Seawater electrolysis presents a groundbreaking opportunity to decarbonize energy systems globally. This method promises a sustainable route to generate hydrogen, a clean energy carrier, directly from seawater. Despite this potential, the approach faces several hurdles that have stifled progress. Issues such as corrosion of electrodes caused by chloride ions, unfavorable chemical reactions linked to chloride, and the hefty expenses associated with effective catalysts create barriers to implementing seawater electrolysis on a wider scale.
Recent research highlights the promising capabilities of self-supported nickel-iron (NiFe) materials. These catalysts emerge as attractive candidates for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) because of their affordability and high intrinsic activity. The use of wood-derived carbon (WC) substrates further enhances these catalysts’ performance owing to their multi-dimensional porous architecture and superior electrical conductivity. These features make WC an excellent platform for optimizing the active sites of NiFe, thus enhancing overall electrochemical performance.
Enhancing Stability through Tungsten Doping
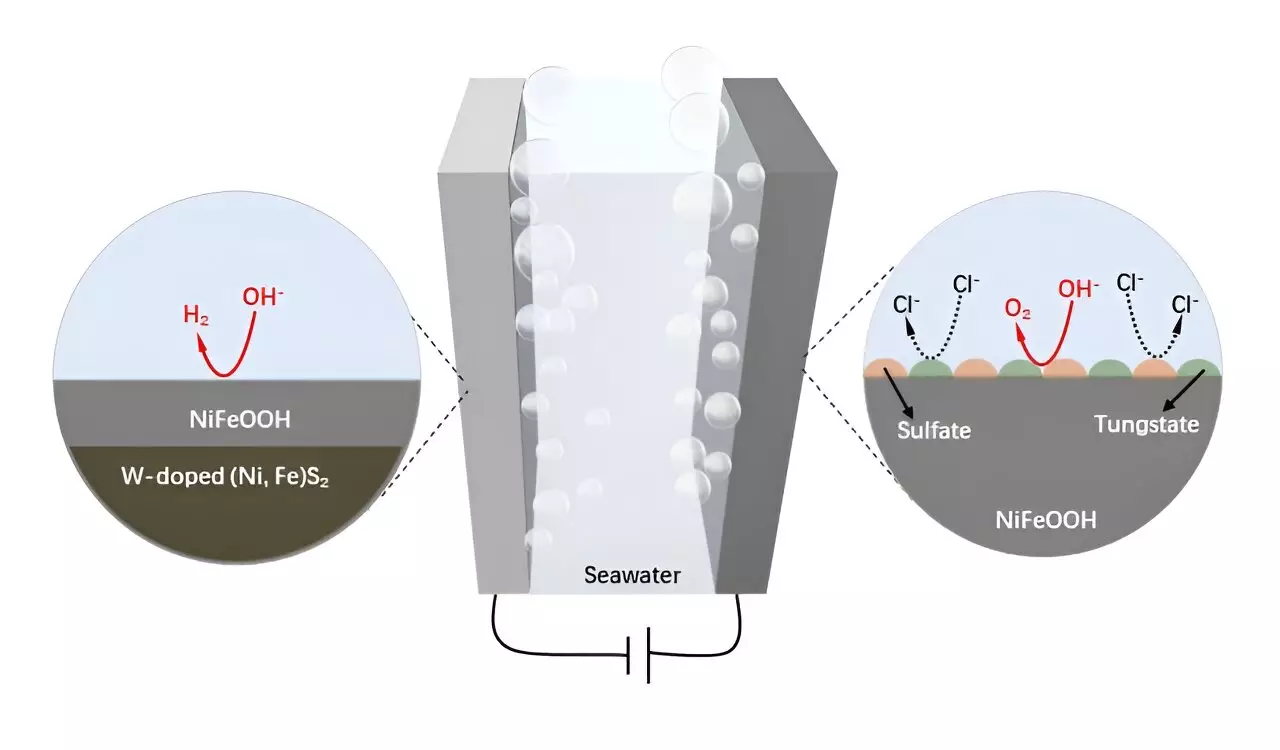
In a significant breakthrough, researchers led by Prof. Hong Chen, along with collaborators from notable institutions like the University of New South Wales and Curtin University, developed a tungsten-doped NiFe sulfide composite supported by wood-based carbon. The study, published in the “Science Bulletin,” presents a novel method that improves the anode stability in corrosive seawater environments. By integrating tungsten into the NiFe framework, the researchers found enhanced resistance to corrosion and higher anode durability, addressing one of the core issues faced in direct seawater electrolysis.
The newly developed W-NiFeS/WC electrode exhibited remarkable structural features, including a three-dimensional hierarchical format equipped with microchannels and tightly anchored nanoparticles. This innovative structure not only optimizes electrical conductivity but also creates an abundance of accessible active sites, improving the overall efficiency of the electrode. The design strategy fosters redox-active centers that significantly boost the electrocatalytic activity in alkaline seawater conditions, thus enhancing performance in both HER and OER when compared to traditional catalysts.
The implications of this research extend beyond enhanced electrochemical performance. By utilizing wood waste in the creation of effective catalysts, this work underscores a commitment to a circular economy. This approach minimizes waste while allowing for sustainable hydrogen production, transforming environmental challenges into opportunities for innovation. The ability to recycle wood waste for energy conversion applications not only promotes efficient use of resources but also aligns with global objectives for green energy and reduced carbon emissions.
This research marks a significant step forward in the field of seawater electrolysis. By overcoming existing challenges through innovative catalyst design and sustainable material use, the potential for hydrogen production from seawater becomes more viable. As the world seeks cleaner energy solutions, advancements like the W-NiFeS/WC electrode signify a promising future in the quest for sustainable hydrogen fuel, demonstrating the intersection of chemistry, material science, and environmental responsibility.

Leave a Reply