The continuous pursuit of improving energy storage technologies has led to a significant focus on solid-state electrolytes, which represent a promising alternative to traditional liquid electrolytes used in conventional batteries. Research by materials scientists at the University of Illinois Urbana-Champaign sheds light on how the structural properties of peptides can enhance the performance of solid-state electrolyte materials. This exploration reveals that the type of molecular configuration, specifically helical structures, can profoundly impact the conductivity and stability of these materials, crucial for the development of next-generation energy storage solutions.
The use of solid-state electrolytes is compelling given their safety advantages over liquid electrolytes. Liquid electrolytes, which facilitate ion movement within batteries, are prone to leakage and combustion, posing inherent dangers. Conversely, solid-state systems aim to eliminate these risks, providing enhanced safety and potential performance benefits. However, achieving high ionic conductivity and thermal stability in these solid materials has been a long-standing challenge in the field. The breakthrough research at the University of Illinois brings forth new mechanisms to address these issues.
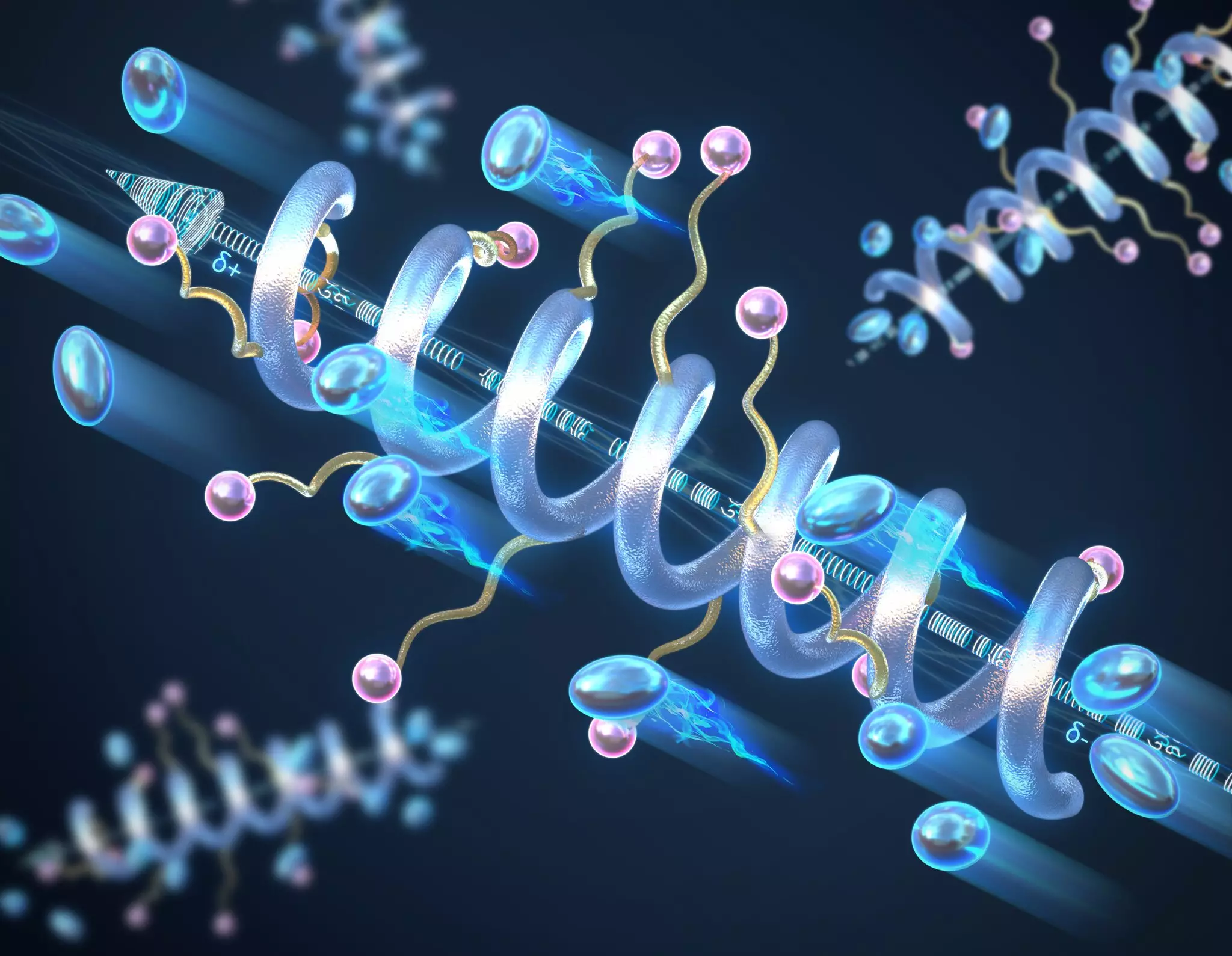
Researchers focused on the helical secondary structure of peptide polymers, identifying its correlation with enhanced ionic conductivity. Unlike traditional polymer configurations that often exist in random coils, the controlled design of helical structures leads to more efficient charge transport mechanisms. The helical arrangement not only gives rise to a substantial macrodipole moment—resulting from the additive effect of smaller dipole moments within each peptide unit—but also escalates the overall dielectric constant. This property is essential, as it measures the polymer’s efficacy in storing electrical energy. The findings indicate that longer helical polymers display a direct relationship with increased conductivity, marking a pivotal advancement in solid-state electrolyte technology.
Stability and Temperature Tolerance
One of the standout features of these helical polymers is their remarkable stability. The research suggests that these structures can withstand higher temperatures and voltages compared to their random coil counterparts without degradation. Professor Chris Evans, who spearheaded the research, emphasizes the robustness of the helical structure, which maintains its integrity even under extreme conditions. This attribute is critical as it supports extended battery life and operational reliability in practical applications, which are paramount in the development of advanced energy storage systems.
Another important aspect of this research is the material’s biodegradability. Peptide polymers can be broken down into their monomer components utilizing enzymes or acidic conditions, promoting environmentally conscious disposal. This attribute not only reduces the ecological footprint of failed batteries but also enables the potential recovery and recycling of materials, a feature increasingly relevant in a world that prioritizes sustainability. By enabling the reuse of starting materials, the research contributes to a circular economy model, aligning with global efforts to reduce waste and material consumption in battery production.
The breakthrough findings regarding the use of helical peptide structures in solid-state electrolytes set the stage for future innovations in energy storage technologies. As researchers continue to refine this approach, the potential applications extend beyond conventional batteries to include electric vehicles, grid storage, and portable electronics. With the growing demand for safe, efficient, and sustainable energy sources, the development of these advanced solid-state electrolytes could revolutionize the battery industry.
The pioneering work conducted at the University of Illinois Urbana-Champaign illustrates how incorporating helical structures into solid-state electrolytes can significantly enhance both performance and sustainability. As the quest for next-generation materials progresses, such insights into material behavior and design will be pivotal in shaping the future of energy storage technologies.

Leave a Reply