The chemical industry is pivotal in producing a myriad of compounds that significantly contribute to various sectors, including pharmaceuticals, agriculture, and materials science. However, the traditional methodologies employed for synthesizing organic molecules heavily rely on organic solvents, leading to considerable environmental harm. As much as 80% of the waste generated in chemical processes is attributed to these solvents, which not only pose disposal challenges but also contribute to toxic emissions and pollution. The shift toward sustainable practices has become a pressing imperative within the chemical manufacturing landscape.
Organic solvents, while facilitating reactions, often introduce complexities. Many substrates and catalysts are susceptible to water, leading to unwanted side reactions when exposed. This reality means that chemists frequently resort to these hazardous solvents, exerting a significant toll on both human health and the environment. The consequences of improper disposal further exacerbate the issue, resulting in soil and water contamination. To navigate these challenges, scientists are increasingly exploring alternatives that promote greener methodologies in the synthesis of organic compounds.
A groundbreaking development in this realm has emerged from the Indian Institute of Science, where researchers from the Department of Inorganic and Physical Chemistry have harnessed agricultural waste to create a bio-based surfactant. This novel surfactant, known as CNSL-1000-M, is synthesized from cashew nut shell liquid (CNSL), a byproduct of the cashew industry, which is notably abundant and inexpensive in India, the world’s second-largest producer of cashews. The team’s initiative exemplifies how agricultural byproducts can not only mitigate waste but also serve as valuable resources in industrial chemistry.
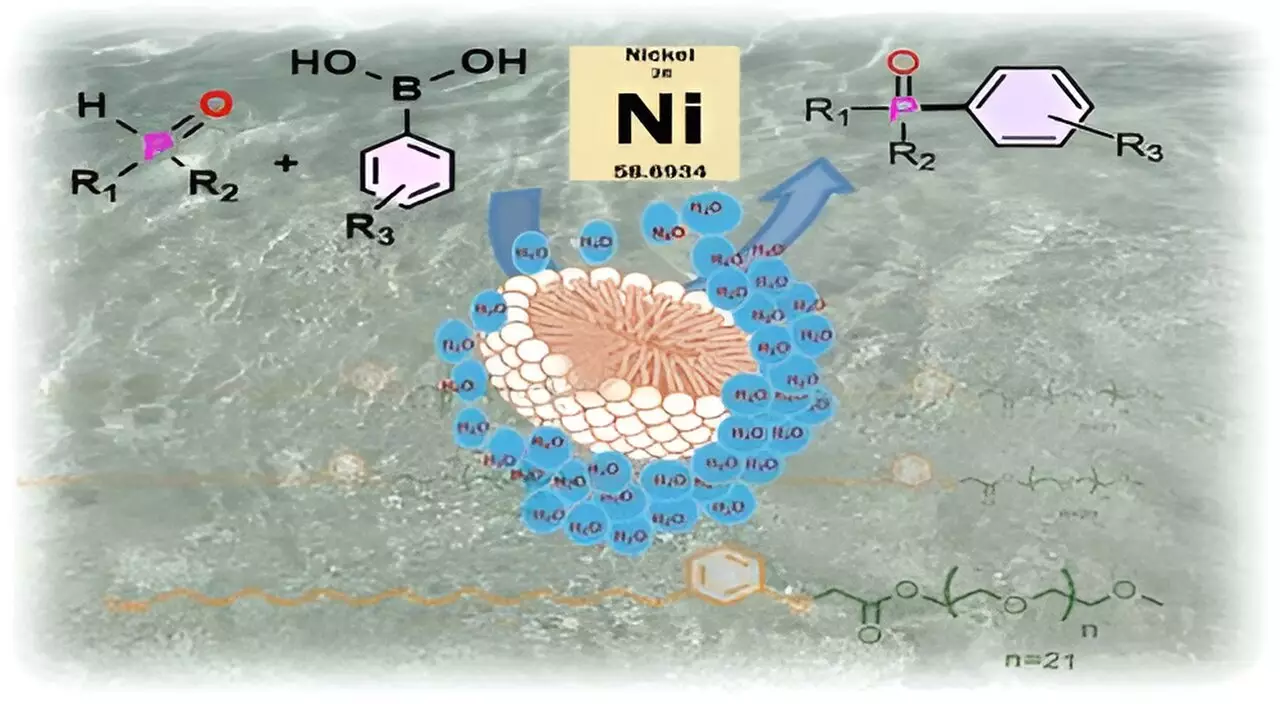
At the core of this innovative approach lies micellar catalysis, a concept that employs surfactants to facilitate chemical reactions in an aqueous environment, providing a shield for sensitive substrates. Surfactants, possessing both hydrophilic and hydrophobic components, self-assemble in water to form micelles—tiny spherical structures that create water-free pockets. Within these protected environments, substrates and catalysts can interact without the detrimental effects of water, much like how a sealed football floats in a body of water without allowing moisture inside. This analogy profoundly illustrates the separation of reactive components from bulk water, creating an ideal setting for chemical transformations to occur.
Utilizing CNSL-1000-M, the research team successfully catalyzed the formation of crucial carbon-phosphorus bonds, an essential step in creating compounds such as the anticancer drug Brigatinib and materials for organic light-emitting diodes (OLEDs). The efficiency of the new surfactant was striking; the yields increased by 80% when reactions were carried out in water compared to traditional organic solvent methods. Furthermore, CNSL-1000-M demonstrated a remarkable 30% higher yield compared to other surfactants previously used in aqueous systems. Notably, the application of this surfactant also permits the use of cheaper nickel catalysts in place of palladium while enabling reactions at lower temperatures.
The researchers emphasize their commitment to fostering collaboration with industries to facilitate the transition from hazardous organic solvents to aqueous micellar technology. This transition is not just about improving yields but fundamentally transforming the sustainability of chemical synthesis. The broader implications of adopting such green technologies extend to reducing ecological footprints, enhancing safety, and promoting environmental stewardship within the chemical industry.
The innovative synthesis of CNSL-1000-M surfactant marks a significant milestone towards sustainable chemistry. As industries increasingly seek methods to mitigate their environmental impact and adhere to stricter regulatory requirements, solutions like micellar catalysis represent a promising path forward. Continued research and industry partnership will be critical in scaling this technology and ensuring a sustainable future for chemical synthesis. The potential of agricultural waste to serve as a catalyst for change underscores the importance of rethinking resource use in our quest for greener alternatives.

Leave a Reply