The journey of innovation often resembles a winding path, filled with missteps and discoveries. Take, for example, Thomas Edison’s quest to develop the incandescent light bulb. His relentless experimentation with various materials, culminating in the selection of tungsten for the filament, laid the groundwork for countless advancements that followed. This method of trial and error continues to be a cornerstone of research in materials science today, particularly in the realm of electrochemical devices like batteries, which play an integral role in our daily lives. However, the pursuit of innovation now encompasses more than mere experimentation; it requires a profound comprehension of material science principles and the complex interplay between the materials’ chemical properties and their physical structure.
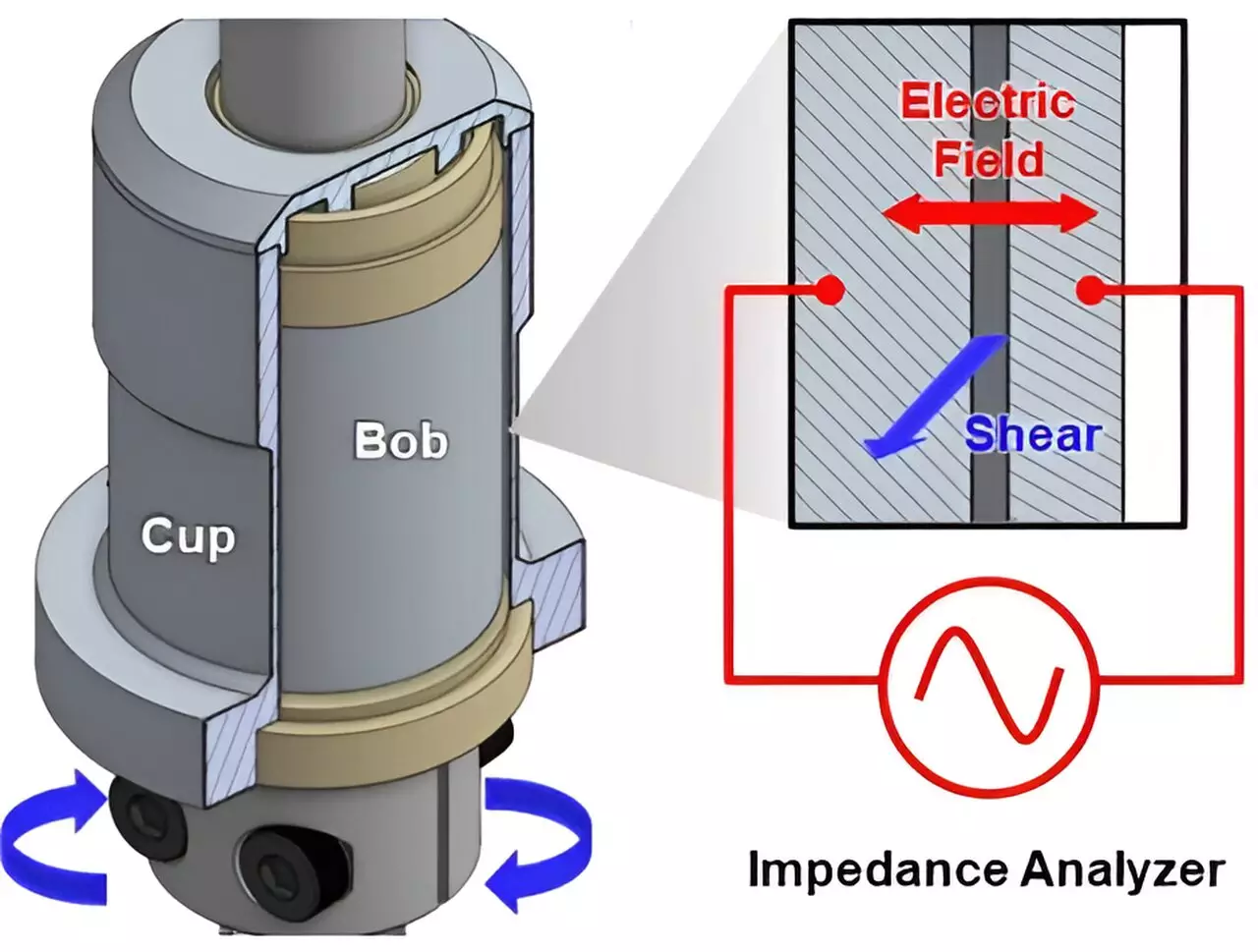
In contemporary research, engineers are tasked with deciphering the intricate behavior of electrons within slurries—viscous mixtures composed of conductive materials used in energy storage devices. A recent study published in the Proceedings of the National Academy of Sciences by a collaborative team from the University of Delaware, Northwestern University, and various industry partners sheds light on this complex subject. The research specifically focuses on how variations in slurry microstructure influence the efficiency of electron transfer, a critical factor in battery performance.
The importance of understanding these materials lies in their fundamental role in electrochemical reactions. When a battery is in operation, electrons must move through these conductive slurries, and the ease of this movement can drastically affect performance outcomes. Thus, researchers aim not only to discover new materials but also to enhance existing ones by optimizing their processes and structures.
Central to this research is the collaboration between eminent scientists, including Norman Wagner from the University of Delaware, who emphasizes the necessity of comprehending how both chemistry and microstructure play a role in device performance. The lead authors, who include professionals from various backgrounds, stress that developing more efficient batteries and other electrochemical devices goes beyond selecting the right chemicals—it involves understanding the materials’ rheology, or how they flow and behave under different conditions.
Researchers found that even in well-designed slurries, the conductivity can vary significantly based on how the components interact at the microscopic level. Visualize a race where multiple racecars are designed to the same specifications; if each car is assembled differently, their performance will inevitably diverge. The same applies to batteries where even subtle adjustments in manufacturing can yield significantly different performance measures.
One of the critical discoveries of this research is how electrons hop between clusters of conductive particles within the slurry. In a traditional conductive system, electrons might move seamlessly. However, in a slurry where particles are suspended rather than solidly connected, the electrons face obstacles that complicate their journey. Wagner’s research utilized advanced techniques, including neutron scattering, to analyze these phenomena in-depth.
The insights gained from this study lead to a broader understanding of not only how conductivity is affected by composition but also how processing techniques can be optimized to improve the conductive properties of slurries. The result is a foundational framework that could guide further research and development in energy storage technologies.
The ramifications of this work extend beyond battery technologies. For instance, understanding the microstructural dynamics of slurries could enhance water deionization techniques or bolster the efficiency of electrolyzers—devices designed to split water into hydrogen and oxygen. In many ways, this research serves as a blueprint for future energy technology innovations, providing avenues for scientists to explore systematically.
Wagner notes that while their findings do not solve a specific battery issue outright, they pave the way for others in the field to explore how variations in material composition and processing can yield more effective electrochemical systems. The overarching theme here is synergistic: achieving breakthroughs in energy storage will require an integrated approach, harmonizing chemical formulation with strategic processing.
In sum, the evolution of materials used in electrochemical devices is not solely a story of discovery but a complex narrative woven with understanding and application. As researchers like Wagner and his colleagues continue to unpack the nuances of material performance, the promise of more efficient energy solutions becomes ever more attainable. Riding on the legacy of pioneers like Edison, today’s engineers stand at the forefront, poised to harness the intricate interplay between structure, process, and chemistry to drive future advancements in technology. The journey is far from over, but armed with knowledge and collaboration, the road ahead is indeed promising.

Leave a Reply