Fragrances have held a significant place in human culture from the earliest civilizations. Our intrinsic desire for pleasant scents has led to the creation and refinement of perfumes across diverse societies. Historically, aromatic compounds were believed to promote health and spiritual well-being, making them highly sought after for ceremonies, daily living, and personal grooming. As this quest for captivating aromas evolved, one particular compound—ambrox—stood out as a prized fragrance ingredient for centuries, synonymous with luxury and elegance. This fascination continues today, evolving alongside advancements in chemical synthesis.
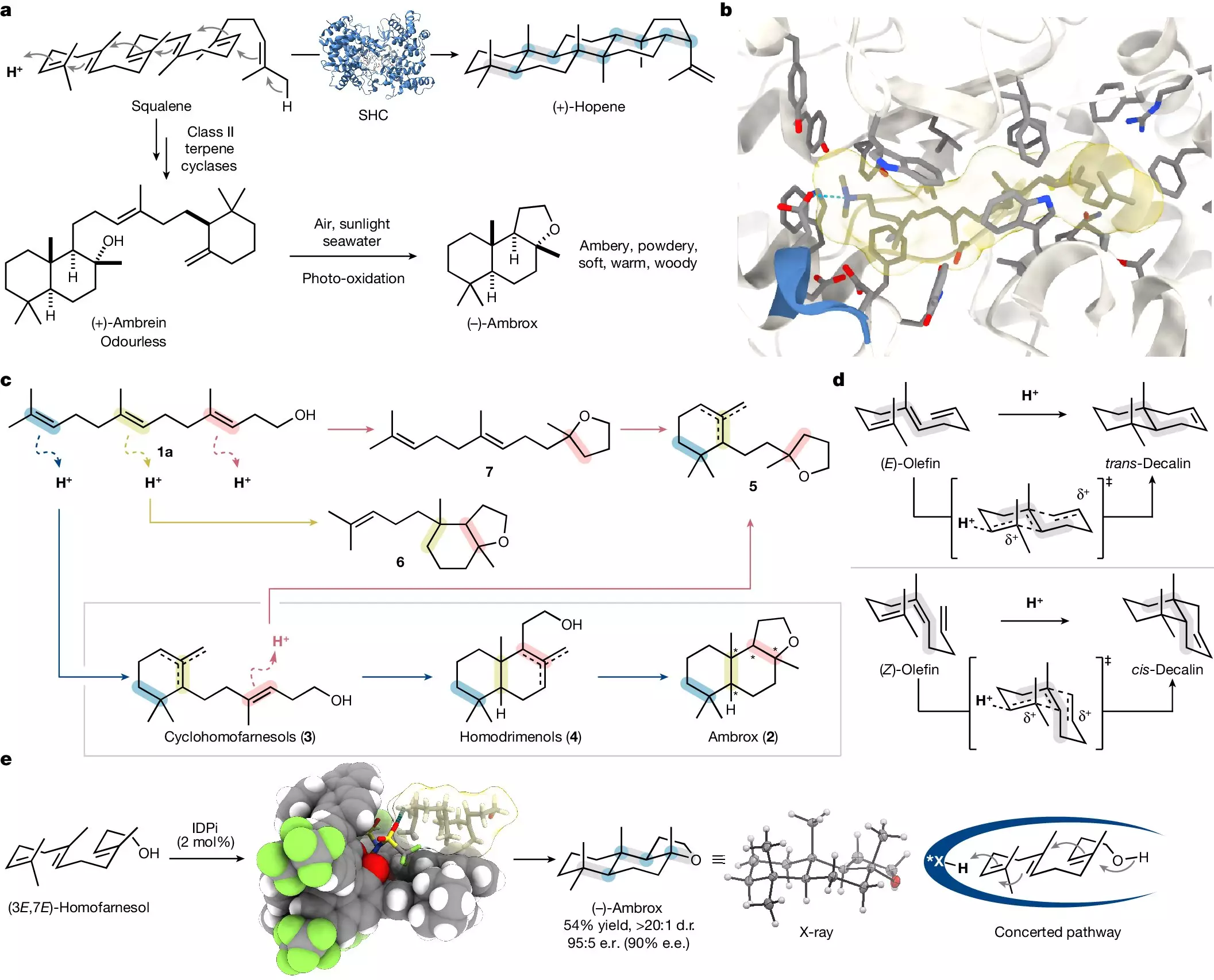
Ambrox, primarily derived from the elusive ambergris produced in the gastrointestinal tract of sperm whales, has long been a staple in the fragrance industry. However, the extraction process is both ethically dubious and ecologically impactful, not to mention the scarcity of this substance in nature. Historically, about 30 tons of ambrox were produced annually; however, the reliance on whale-derived resources raised sustainability concerns. Furthermore, the chiral nature of ambrox presents additional challenges since only one of the 16 possible molecular structures carries the adored scent. This necessitated the development of stereoselective synthesis processes to isolate the correct isomer.
Fortunately, advancements in organic chemistry enabled researchers to locate alternative sources. A well-known method involves partial synthesis from (-)-sclareol, a natural compound found abundantly in clary sage. Yet, this plant-based method comes with limitations such as dependence on seasonal availability and multi-step processes, often complicating large-scale production.
In a recent significant advancement, a team of researchers, led by Prof. Benjamin List of the Max Planck Institute for Coal Research, has developed a novel laboratory-based synthesis method for producing ambrox that bypasses the lengthy ecological concerns tied to whale extraction and plant dependability. Their work, heralded in the journal Nature, demonstrates a streamlined approach utilizing a catalytic asymmetric polyene cyclization of homofarnesol—an exciting innovation that promises not only efficiency but also high selectivity in producing the desirable (-)-ambrox isomer.
According to Mathias Turberg, a lead author and doctoral student within the team, the inspiration came from biological mechanisms where nature efficiently transforms simple compounds into complex molecules in a singular step. This objective—to replicate nature’s efficiency—has been a driving force in modern chemistry, pushing researchers to seek ways to simplify synthetic pathways while maintaining environmental sustainability.
The Mechanism: Imitating Nature’s Efficiency
The synthesis involves the versatile C15 building block, nerolidol, readily sourced from various plants or produced on a larger scale. The collaborative efforts with BASF facilitated the conversion of nerolidol to the C16 compound, homofarnesol, which is then selectively transformed into (-)-ambrox using a sophisticated acidic catalyst in a fluorinated solvent. Dr. Na Luo, the study’s primary author, emphasizes the crucial role of the dual components in ensuring that the synthesized product is of high purity and selectivity.
The team’s method stands out due to its remarkable rapidity and efficiency: while conventional biocatalysis demands three to four days, their approach produces results overnight. The confinement of the catalyst and the use of a specialized solvent ensure that the process is not only quick but also allows for the recovery and reuse of materials, pointing toward potential industrial applications.
Future Implications and Industrial Applications
This innovative synthesis technique signifies a promising future for the fragrance industry, where sustainability and efficiency are increasingly prioritized. The ability to generate (-)-ambrox on-demand, alongside the reusability of catalysts and solvents, offers a sustainable alternative to traditional methods that have long relied on resource-intensive and ethically questionable practices.
As research continues to refine this process, it is evident that the field of fragrance production is evolving into a more ethical and environmentally conscious arena. Prof. List’s group represents not just a technological advancement but a paradigm shift in how we perceive and obtain the scents that enrich human experience. The implications stretch far beyond personal fragrances, offering a window into responsible sourcing and production in the broader chemistry landscape.

Leave a Reply