Fuel cells have emerged as promising alternatives to conventional energy sources, generating electricity through electrochemical reactions while circumventing the detrimental effects of combustion. This technology holds the potential to power a multitude of applications, including electric vehicles, portable electronics, and large-scale industrial machinery. However, despite their environmental benefits, the high cost of materials and reliance on precious metal catalysts have hampered their global adoption. In light of these challenges, anion-exchange-membrane fuel cells (AEMFCs) introduce an innovative approach by utilizing economically viable and abundantly available materials. Recent strides in research suggest that these cells can overcome many barriers, enhancing their feasibility for widespread commercial deployment.
Challenges with Current Fuel Cell Technologies
Many contemporary fuel cell models are predominantly based on precious metals, primarily platinum, which significantly inflates production costs and limits scalability. The issue is compounded by limited availability of these materials, creating a supply chain concern that might hinder future advancements in fuel cell technologies. Researchers have been keenly aware of the pressing need for alternatives and have been exploring the potential of non-precious metal catalysts in AEMFCs. However, an alarming finding across various studies has been the predilection of these alternative materials to self-oxidation under operational conditions, leading to irreversible failure and loss of efficiency.
The key to unlocking the full potential of fuel cells lies in developing catalysts that not only perform adequately but also maintain stability during continuous operation. Recent research initiatives demonstrate hopeful progress towards this end. Notably, efforts from scholars at Chongqing University and Loughborough University have brought forth a groundbreaking solution that addresses the prevalent weaknesses of non-precious metal catalysts in AEMFCs.
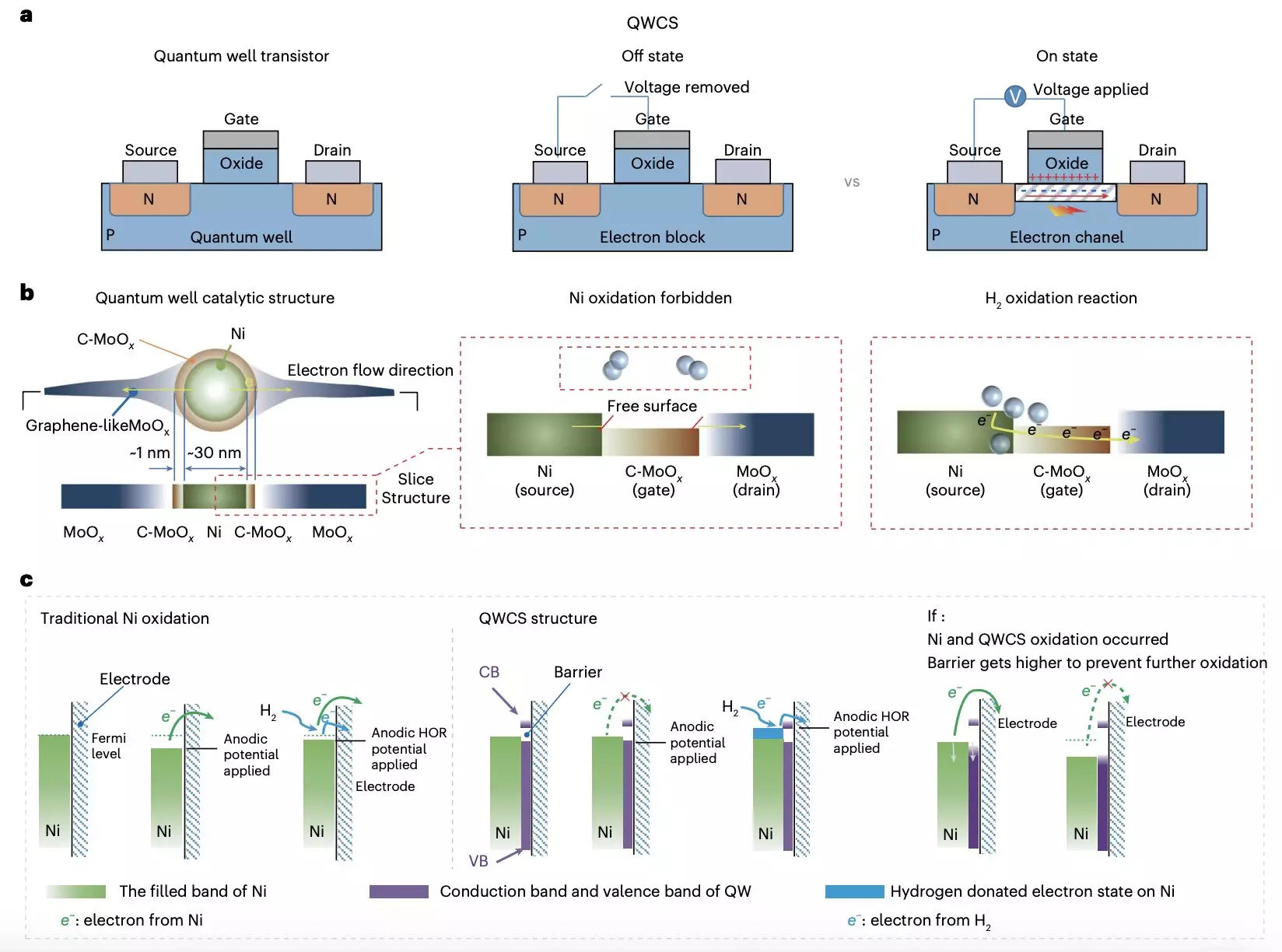
The innovation centers around a novel concept known as the quantum well-like catalytic structure (QWCS). This sophisticated nanostructure comprises quantum-confined metallic nickel nanoparticles encapsulated within a heterojunction of carbon-doped molybdenum oxide (C-MoOx) and amorphous MoOx. The unique architecture of the QWCS allows for the selective transfer of electrons produced from hydrogen oxidation reactions while mitigating the risk of self-oxidation of the nickel nanoparticles.
The research, led by Yuanyuan Zhou and Wei Yuan, details a mechanism by which this QWCS can effectively safeguard the metallic nickel from degradation, allowing for a prolonged operational life of the fuel cells. The carefully designed arrangement within the QWCS plays a critical role in maintaining electron transfer, enabling the nickel-based catalyst to withstand external challenges such as electro-oxidation.
The efficacy of the QWCS has been substantiated through rigorous testing, showcasing its exceptional stability—surviving over 100 hours of continuous operation in demanding environments. This stability translates into practical applications, with the demonstration of an anode-catalyzed alkaline fuel cell achieving a remarkable specific power density of 486 mW/mgNi—an impressive metric indicative of its performance potential. Moreover, the fuel cell’s resilience during shutdown-start cycles further underlines the operational reliability of the newly designed catalyst.
Foundational to this advancement is the QWCS’s capacity to maintain electron barriers, allowing electrons produced through hydrogen oxidation to cross into the energy valley with relative ease while preventing unwanted electron transfer from the nickel itself. This delicate balance ensures that the catalyst remains functional over an extended period, representing a significant stride towards cost-effective and efficient AEMFC technology.
With the insights gleaned from the research team’s findings, it is evident that QWCS technology could forge a new path in fuel cell development. As researchers continue to explore ways to integrate quantum confinement into catalyst design, the feasibility of non-precious metal fuel cells will only increase. This work is not merely a technological triumph; it symbolizes a shift towards sustainable energy solutions that could redefine the future of how we harness energy, offering a cleaner and economically viable alternative to fossil fuel dependency. The implications extend beyond just fuel cells, potentially influencing a vast array of catalyst applications across various fields, setting the stage for innovation that aligns with global sustainability goals.

Leave a Reply