In recent years, the complexities of Alzheimer’s disease have led researchers down an increasingly convoluted path, one where the traditional understanding of amyloid fibrils as mere villains in the narrative of neurodegeneration is being called into question. While amyloid fibrils have long been the focus of drugs aimed at reducing their levels, emerging evidence suggests that these protein structures might play a more sophisticated role than once thought. Instead of being direct causative agents of Alzheimer’s, amyloid fibrils may serve as adaptive responses to oxidative stress and other environmental challenges faced by the human brain.
The longstanding assumption that high quantities of amyloid definitively lead to dementia has been undermined by findings showing that many individuals with substantial amyloid deposits do not exhibit symptoms of cognitive decline. Such inconsistencies raise critical questions regarding the existing treatment strategies, primarily those targeting amyloid reduction. The urgency for a paradigm shift in dementia research has never been clearer.
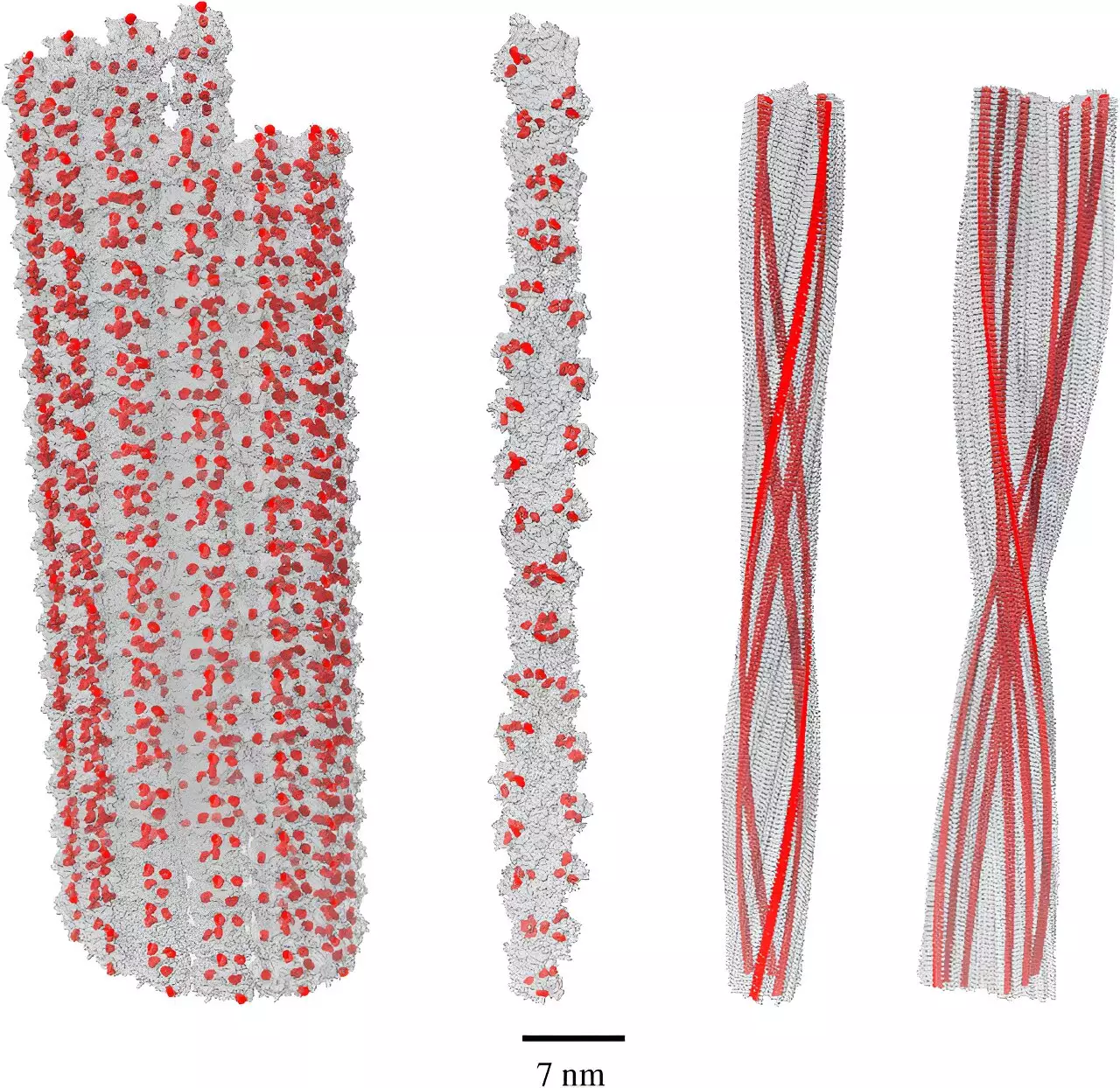
A pivotal shift in perspective comes from the realm of quantum biology. Recent advancements led by Dr. Philip Kurian and his team at Howard University’s Quantum Biology Laboratory have unveiled the profound interplay between quantum effects and biological systems, particularly in the context of amyloid fibrils. Their research highlights a phenomenon known as single-photon superradiance—a mechanism where molecules collectively manage to absorb harmful high-energy photons and re-emit them at safer energy levels. This discovery not only enhances our understanding of amyloid structure but also suggests a protective rather than pathological role of these fibrils in neurobiology.
Kurian’s findings indicate that amyloid fibrils exhibit an extraordinary capability to harness superradiant effects due to their dense arrangement of tryptophan amino acids. This configuration grants amyloid structures the ability to downconvert damaging energy from free radicals, effectively mitigating oxidative stress. The realization that amyloid can function as a bio-protective agent in an otherwise hostile biochemical environment transforms the discussion surrounding Alzheimer’s treatment strategies, emphasizing the need to reconsider biochemical pathways in light of quantum effects.
Oxidative stress has emerged as a critical concern in the conversation about Alzheimer’s disease. It refers to the cellular damage inflicted by an excess of free radicals, which not only hampers ordinary cellular functions but also leads to significant brain deterioration over time. With an acknowledged connection between oxidative stress and Alzheimer’s, understanding how amyloid interacts with such stressors can offer transformative insights into disease progression and treatment.
Dr. Kurian’s research brings a revolutionary thought to the forefront: amyloid could actually be a countermeasure employed by the body to deal with heightened oxidative conditions, particularly the influx of damaging UV photons. This interpretation compels researchers to reframe their approaches, diverting focus from solely diminishing amyloid levels to understanding the holistic functions these fibrils perform within the body’s larger system.
The implications of Kurian’s work echo throughout the scientific community, stirring enthusiasm among researchers who may have previously overlooked the potential evolutionary advantages of amyloid fibrils. Professor Lon Schneider, a noted figure at the USC California Alzheimer’s Disease Center, underscores the vast horizons of understanding that this new perspective opens up. By suggesting that amyloid aggregation could be an evolutionary safeguard, rather than a straightforward pathological process, researchers are now prompted to rethink their strategies and frameworks for treating dementia.
A shift to considering quantum biological principles provides a richer, more nuanced understanding of living systems. As Kurian himself notes, this opens doors for interdisciplinary collaboration between fields that have historically operated in silos, such as quantum physics, computational biology, and neuroscience.
As promising as these findings are, the next steps for Kurian and his team involve validating these predictions through further experimentation. The academic community is urged to embrace this fresh lens, as it could catalyze a wave of innovative treatment strategies that align with the complexities of Alzheimer’s disease.
The work done by Kurian and his group serves as a vital reminder that biological research is a continuously evolving narrative, one that intersects with multiple scientific disciplines. In doing so, it also reinforces the idea that true scientific progress often requires the courage to upend entrenched beliefs and to seek knowledge from various fields of study. Through exploring these uncharted territories, a clearer vision of Alzheimer’s could emerge—one that prioritizes understanding over assumption, and adaptation over eradication.

Leave a Reply