Artificial intelligence (AI) has emerged as an invaluable asset across numerous fields, including chemistry. However, a major limitation persists—the inability to elucidate the reasoning behind AI decisions, often referred to as the “AI black box.” This challenge presents a significant roadblock for chemists who rely on AI to optimize chemical compounds but struggle to understand the underlying principles that inform those optimizations. Recently, a pioneering effort at the University of Illinois Urbana-Champaign has made significant strides in addressing this issue, focusing on the development of improved molecules for harvesting solar energy. Through an innovative approach that marries AI with automated chemical synthesis and experimental validation, researchers have unlocked vital insights into the chemical properties that contribute to molecular stability.
The Interdisciplinary Team Making Waves
The breakthrough at the University of Illinois was driven by a collaborative, interdisciplinary team, co-led by notable professors including Martin Burke from the chemistry department, Ying Diao from chemical and biomolecular engineering, Nicholas Jackson in chemistry, and Charles Schroeder from materials science and engineering. Their work was further bolstered by collaboration with Alán Aspuru-Guzik from the University of Toronto. The collective efforts of these scientists culminated in a study published in the journal *Nature*, revealing a significant leap in understanding the chemical foundations that contribute to more robust light-harvesting molecules.
From Optimization to Understanding
Historically, the primary focus of AI in chemistry has been optimization—enhancing molecular characteristics without elucidating the reasons behind those enhancements. As Professor Jackson noted, the frustration stems from AI’s ability to suggest improvements that lack explanation, leaving researchers in the dark about crucial chemical properties and functions. The pioneering initiative undertaken by the Illinois team not only served to optimize these molecules but also endeavored to demystify them. By transforming the AI black box into a transparent construct, they successfully identified the underlying factors that contribute to photostability in light-harvesting materials.
The impetus for this research was a longstanding challenge in the field of organic solar cells, which utilize flexible materials rather than the traditional silicon-based panels proliferating across the globe. As Professor Diao pointed out, the stability of these organic materials has been a critical hindrance to their commercialization, a problem that has lingered since the 1980s. While organic photovoltaics offer numerous advantages, including the ability to convert a broader spectrum of light—including heat and infrared—into energy, their vulnerability to degradation when exposed to light has posed significant limitations.
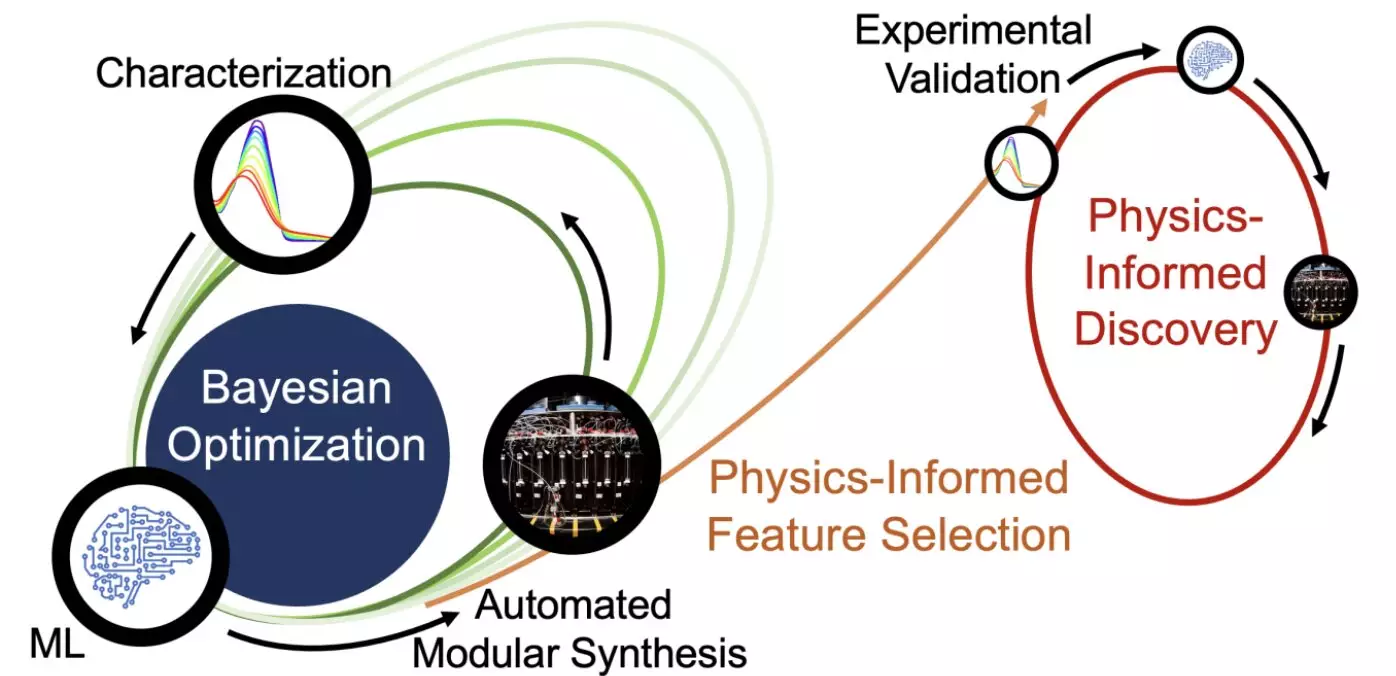
At the heart of the researchers’ methodology lies the “closed-loop transfer” approach, initiated with an AI-guided optimization protocol called closed-loop experimentation. This innovative system requested the AI to enhance the photostability of specific light-harvesting molecules. Following this directive, the AI algorithm provided hypotheses related to the types of chemicals to synthesize, which were then put through a series of iterative rounds of synthesis and experimental validation. This iterative process resulted in the development of 30 new chemical candidates, showcasing the power of combining modular chemistry with automated synthesis.
Discovering Stability through AI-Enhanced Insights
Unlike conventional AI-led approaches that conclude with simply identifying high-performing products, the closed-loop transfer method sought to unearth the hidden principles responsible for increased stability. As the synthesis experiments progressed, parallel algorithms analyzed the resulting molecules, enabling the development of predictive models around their chemical features. These insights resulted in testable hypotheses, facilitating new avenues of discovery in the quest for understanding photostability.
A Proof of Principle and Future Directions
To validate their hypotheses concerning photostability, the researchers embarked on a detailed examination of three distinct light-harvesting molecules, targeting a specific high-energy region identified during previous synthesis phases. They successfully demonstrated that the appropriate solvent selection could enhance molecular stability by up to four times. This discovery not only serves as a compelling proof of principle for their innovative methodologies but also suggests broad applications across diverse material systems.
The work undertaken by the interdisciplinary team at the University of Illinois presents a transformative paradigm in materials development, opening the door for future investigations that rely on synergistic relationships among disciplines. The potential for AI to generate actionable hypotheses marks a significant step forward in addressing longstanding challenges in chemistry and materials science. Ultimately, this research exemplifies the promising trajectory of AI when integrated with experimental validation, offering a glimpse into a future where scientists can readily explore the vast landscape of chemical possibilities with augmented understanding and insight.

Leave a Reply