In the realm of battery technology, particularly in rechargeable batteries, the interaction between electrodes and electrolytes plays a pivotal role in determining efficiency and performance. A significant focus of recent research has been on enhancing this electrode/electrolyte interface to improve the energy density, especially in lithium-metal batteries (LMBs). These advanced batteries utilize lithium metal anodes instead of the conventional graphite constructs of lithium-ion batteries (LiBs), which presents an opportunity for substantially increased energy density and faster charging capabilities. However, despite their promise, LMBs face several daunting hurdles that need urgent address.
One of the primary challenges plaguing LMBs is the formation of lithium dendrites during the charging process. These dendrites are micrometer-sized, tree-like structures that emerge on the surface of the anodes. Their development not only impairs the performance of the battery but also elevates the risk of short circuits, potentially leading to overheating or even catastrophic fires. Additionally, high production costs and low Coulombic efficiency further encumber the commercialization of LMB technology. The quest to overcome these obstacles has led researchers to investigate novel approaches to stabilize the electrolyte and improve the interactions at the interface.
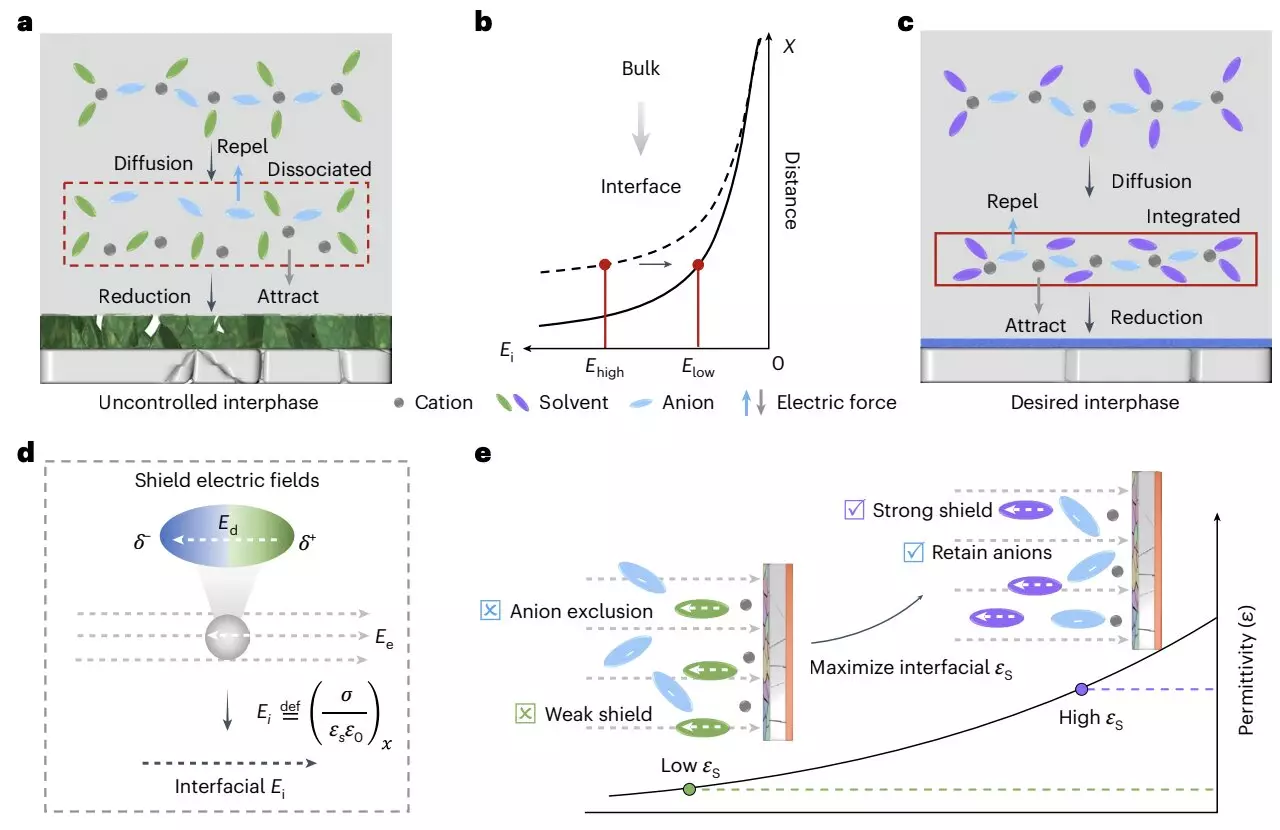
A recent study by researchers from Zhejiang University and other institutions published in *Nature Energy* ventured into a new paradigm by emphasizing the role of the dielectric environment in stabilizing the electrode/electrolyte interface. The researchers introduced a dielectric protocol aimed at boosting the stability and safety of LMBs. Their method suggests that by carefully regulating the dielectric medium, it is possible to enhance the integrity of cation-anion coordination, which is essential for the formation of the solid-electrolyte interphase (SEI).
According to co-author Xiulin Fan, this dielectric protocol ensures that the cation-anion pairs remain intact while allowing for better exposure of the electrolyte to an interfacial electric field. By employing a non-solvating solvent with a high dielectric constant, the researchers were able to create an environment conducive to maintaining the stability of these pairs, which in turn fosters robust interfacial chemistry between lithium deposits and the electrolyte.
The proposed mechanism leverages a periodic oscillatory distribution of cation-anion pairs at charged interfaces, which can significantly influence the battery’s performance. The reduction of oscillation amplitude leads to detrimental electrolyte decomposition and elevated surface impedance, conditions that are not favorable for battery longevity.
By sustaining a high oscillation amplitude while maintaining cation-anion coordination, the dielectric protocol effectively addresses these issues. The researchers successfully tested this approach with an ultra-lean electrolyte, achieving an impressive energy density of 500 Wh/kg in lithium-metal pouch cells. This energy density far exceeds that of traditional LiBs, substantiating the protocol’s potential for advancing battery technology.
The findings from this research not only provide a framework for improving lithium-metal battery performance but also lay the foundation for further innovations in electrolyte design. Researchers and developers are likely to derive inspiration from these findings, aiming to create other advanced electrolytes using the dielectric-mediated approach. This could lead to the commercialization of more reliable, high-density battery solutions, addressing the escalating demand for efficient energy storage devices in the electric vehicle and renewable energy sectors.
Furthermore, as highlighted by Fan, addressing safety concerns remains a paramount consideration. Enhancing energy density through improved interfacial chemistry carries a responsibility to mitigate the associated risks of fires and explosions that arise from high-capacity battery systems.
Advancing lithium-metal battery technology demands an ongoing commitment to innovative research focused on the intricate dynamics of electrode/electrolyte interfaces. The work done by the researchers at Zhejiang University presents an exciting step towards overcoming the existing limitations of LMBs, potentially reshaping the future of energy storage technology. As the market for electric vehicles and sustainable energy storage continues to flourish, so does the urgency for breakthroughs that promise both enhanced performance and safety. Through concerted efforts and continued exploration, the goal of achieving a high-energy-density battery capable of supporting a low-carbon economy appears more attainable than ever.

Leave a Reply