Recent advancements in nuclear fuel technology have highlighted the unique chemistry dynamics of molten uranium trichloride (UCl3), documented in a groundbreaking study published in the *Journal of the American Chemical Society*. This pioneering research opens doors for future nuclear reactors, which are positioned to become essential in the global endeavor for sustainable energy solutions. Researchers, including Santanu Roy from Oak Ridge National Laboratory (ORNL), emphasize the significance of understanding the microscopic behaviors of high-temperature liquid fuels, which are pivotal for designing advanced reactors. This paper represents a crucial leap forward, potentially influencing reactor safety and efficiency for decades to come.
Throughout the past half-century, molten salt reactors (MSRs) have emerged as promising contenders for efficient nuclear energy production. Initial prototypes from ORNL during the 1960s demonstrated the viability of this technology; however, renewed interest has surged in the wake of global warming concerns and the urgency for decarbonization. As many countries recommit to nuclear power as part of their energy strategies, understanding the behavior of liquid fuel salts like UCl3 becomes imperative. The distinct behavior of these salts, which differ fundamentally from solid uranium dioxide used in conventional reactors, is essential to efficiently harness their potential as fuel sources.
The chemistry associated with actinide series elements, particularly uranium, poses formidable challenges for scientists. Most significantly, the high melting points of these salts demand experimental techniques that can operate under extreme conditions. As noted by researchers, elucidating the atomic-level behavior of liquid UCl3 involves unraveling intricate behaviors influenced by high temperatures and complex ion interactions. This is particularly true given that these molten salts maintain unique chemical bonding properties when passed from solid to liquid states.
Innovative Research Methods Utilized
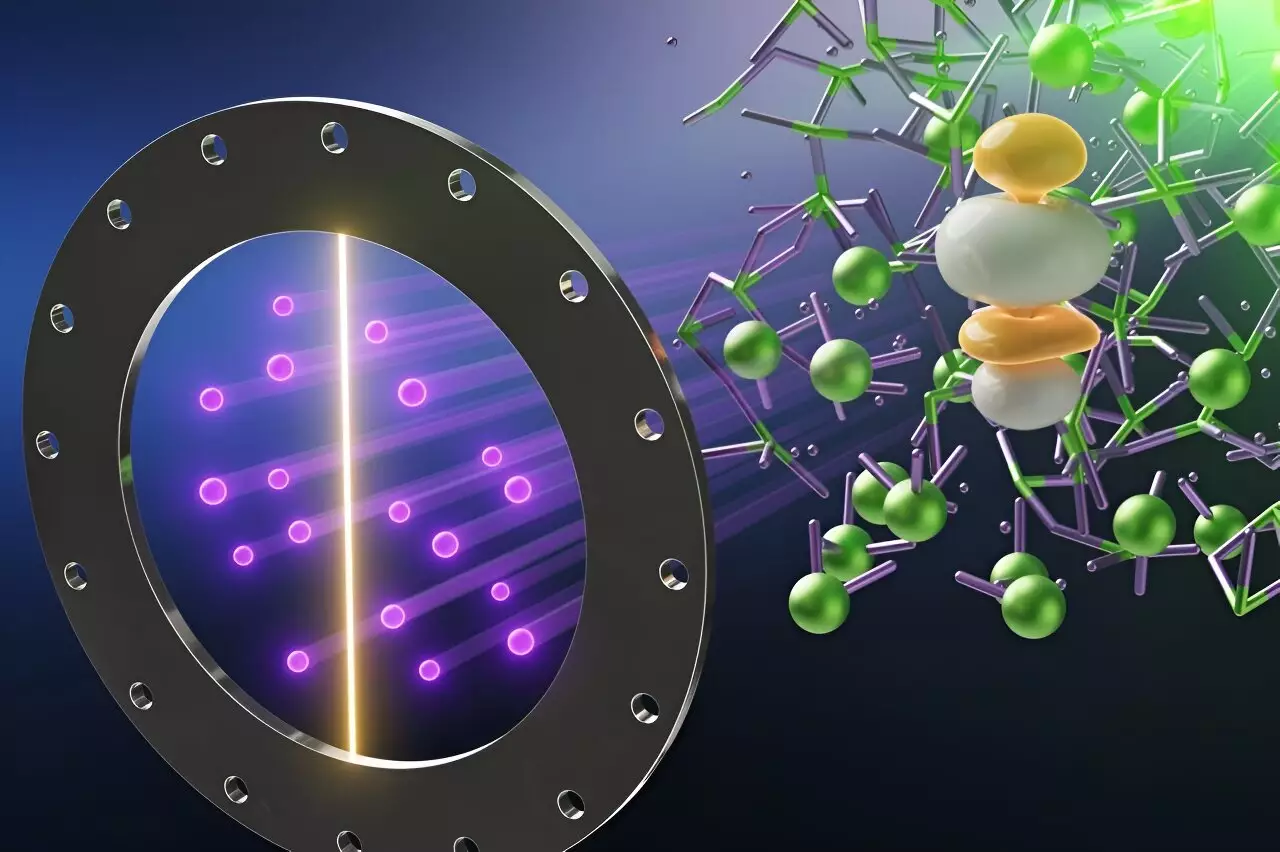
The collaborative effort between ORNL, Argonne National Laboratory, and the University of South Carolina has pioneered a multidisciplinary approach that combines computational models with state-of-the-art experimental methods. The Spallation Neutron Source (SNS), recognized as one of the brightest neutron sources worldwide, proved invaluable for conducting high-precision neutron scattering experiments. By directing neutron beams at UCl3 in its molten state, researchers achieved groundbreaking measurements of its chemical bonds, revealing unexpected behaviors that challenge established chemical conventions.
Neutrons interact with the atomic nuclei, allowing scientists to observe and map the intricate dynamics of material at a fundamental level. This unprecedented insight has provided crucial data regarding atomic positions, movements, and magnetic properties—information essential for refining future practices in the design of reactors.
What emerged from the study was a profound revelation regarding the bonding behavior within molten UCl3. Contrary to conventional expectations, bond lengths between uranium and chlorine shortened as the material transitioned to its liquid state. This unexpected process defies the typical thermal expansion observed in most substances and shines a light on the complex and often chaotic behavior of materials in extreme environments.
Additionally, researchers found that bond lengths fluctuated in an oscillating manner, sometimes stretching to lengths much greater than those observed in solid UCl3, while at other times contracting to remarkably short distances. The discovery that these oscillating patterns occur at extraordinarily high speeds—on the order of one-trillionth of a second—pushes the boundaries of our comprehension regarding chemical bonding behaviors in extreme conditions.
The ramifications of these findings extend far beyond mere curiosity; they directly inform efforts to create more efficient nuclear reactors. By integrating insights gained about the atomic structure and bonding dynamics of UCl3, scientists can improve both experimental techniques and computational models pertinent to future reactor design. Additionally, this research fosters a deeper understanding of fundamental actinide chemistry, which could have significant implications for addressing issues related to nuclear waste management and pyrotechnology.
The in-depth investigation of molten uranium trichloride marks a significant milestone within the field of nuclear fuel chemistry. As the energy landscape increasingly pivots towards sustainable alternatives, the implications of this research extend into the future of nuclear technology. With the knowledge gained from this study, scientists are not only better equipped to design next-generation reactors but have also laid the groundwork for advancing our overall understanding of actinide chemistry. Such breakthroughs will be crucial in propelling nuclear energy into a new era of safety, efficiency, and ecological responsibility.

Leave a Reply