In the realm of chemistry, the quest for sustainable and energy-efficient methods of synthesis is becoming increasingly critical. One recent development that stands out in this pursuit is the innovative use of dinitrogen (N₂), a molecule that constitutes approximately 78% of Earth’s atmosphere. A research team at the RIKEN Center for Sustainable Resource Science has made significant strides in utilizing this abundant resource for the synthesis of valuable chemical compounds. Their findings, published in *Nature*, explore a novel pathway that could revolutionize the production of alkyl amines—a class of compounds fundamental to pharmaceuticals and polymers—overcoming the traditional barriers associated with dinitrogen’s use.
Despite its abundance, directly leveraging dinitrogen in chemical reactions has long been regarded as a daunting challenge for chemists. The primary hurdle lies in its strong triple bond, which must be broken to initiate any useful chemical reaction. Current industrial practice typically involves the Haber–Bosch process to convert dinitrogen into ammonia before further transforming it into desired products. This multi-step process not only adds layers of complexity but also consumes substantial amounts of energy, rendering it less than ideal in terms of efficiency.
Takanori Shima, a leading researcher on this project, emphasizes the drawbacks of current methodologies, stating that the energy-intensive nature of producing essential alkyl amines underscores the need for more sustainable alternatives. The ideal solution would be the direct utilization of dinitrogen and alkenes in mild conditions to synthesize alkyl amines more efficiently.
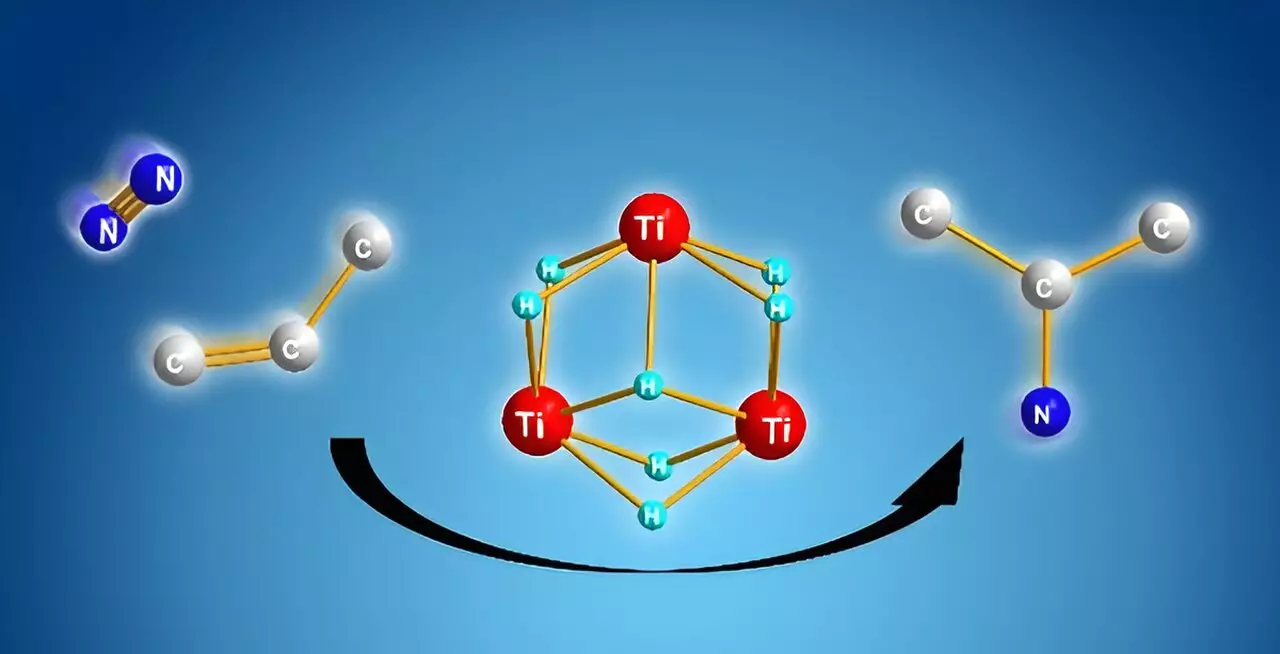
The breakthrough achieved by Shima and his colleagues lies in their previous work on titanium polyhydrides—unique complexes that effectively bridge titanium atoms using hydrogen. This powerful framework was initially recognized for its ability to engage with stable small molecules, like benzene and, importantly, dinitrogen. By employing titanium polyhydrides, the research team uncovered a mechanism that allows for the direct reaction of dinitrogen with alkenes, enabling the generation of alkyl amines without the energy-intensive intermediate steps typically required.
Shima explains, “Our method exploits the cooperative behavior of titanium–hydride units to cleave dinitrogen and form new nitrogen–carbon bonds directly.” This describes a significant departure from conventional approaches, allowing for more streamlined synthesis.
The innovation does not merely lie in the use of titanium polyhydrides; it also hinges on the reaction mechanism. Initially, when alkenes interact with these titanium complexes, they are activated, leaving behind several titanium–hydride units. When dinitrogen is subsequently introduced, these free units interact cooperatively, breaking the strong N≡N bond and facilitating the formation of alkyl amines.
Computational analysis played a pivotal role in this research, revealing that the nitrogen–carbon bond formation is highly favorable energetically compared to other possible reactions, such as nitrogen–hydrogen or carbon–hydrogen bond formations. This critical insight not only confirms the efficiency of the synthetic pathway but also paves the way for further exploration to enhance this reaction into a catalytic process.
Shima’s team’s breakthrough opens up the door for new methodologies in chemical synthesis that prioritize energy efficiency and sustainability. By making direct use of dinitrogen, they have taken a significant step towards reducing energy consumption in the synthesis of key industrial compounds. As research continues, the goal will be to refine this process and expand its applicability. The prospect of transforming traditional chemical pathways using readily available resources like dinitrogen signals a promising shift in how future chemists can approach the synthesis of vital compounds.
The advancement in harnessing dinitrogen for alkyl amine synthesis exemplifies the innovative spirit of contemporary chemistry. As researchers like Shima and his colleagues continue to pioneer new techniques, the hope is that these discoveries will lead not only to more efficient production methods but also contribute to a more sustainable future for chemical manufacturing.

Leave a Reply