The process of photocatalysis is fundamentally driven by the ability of certain materials to harness sunlight to fuel chemical reactions, a phenomenon that draws inspiration from nature’s own mechanism of photosynthesis. This innovative technology has gained traction across various fields, including environmental remediation, chemical manufacturing, and renewable energy production due to its potential for energy efficiency and sustainability. However, an essential prerequisite for photocatalytic processes to gain widespread adoption is achieving high quantum efficiency in light-induced transformations. This efficiency ensures that reactions occur not just rapidly but also under conditions that resemble practicality in industrial settings.
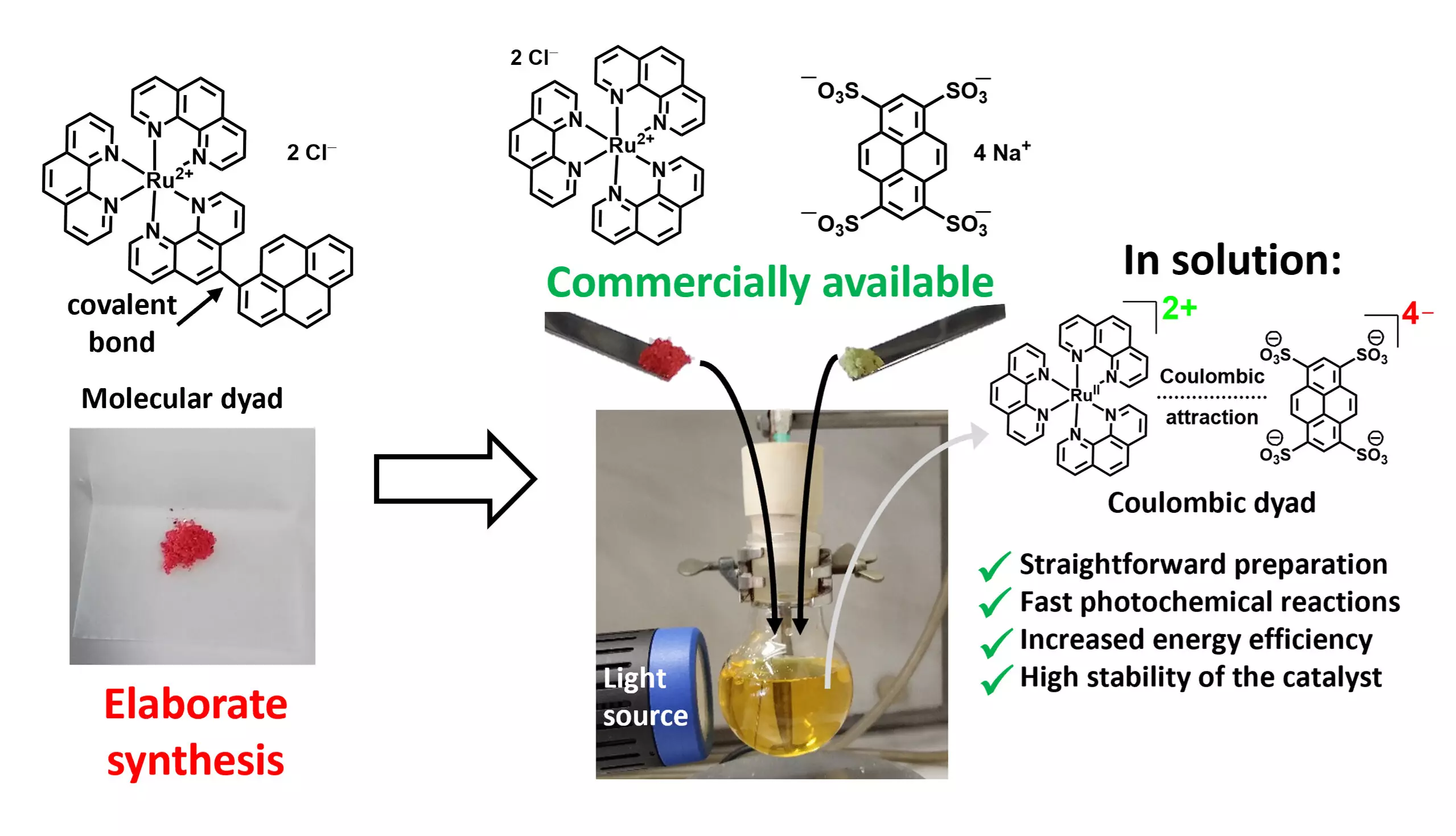
Traditionally, the development of photocatalysts with superior performance has relied on complex synthesis methods, often struggling with high production costs. The ideal photocatalysts are typically composed of two active units connected by a covalent bond, forming what researchers refer to as molecular dyads. The intricate preparation of these dyads, characterized by a multi-step synthesis process, poses a significant barrier to their large-scale applications. Current methodologies often hinge on the use of precious metals, such as iridium or ruthenium, known for their outstanding catalytic properties but burdened by economic limitations.
In a significant breakthrough, a research team led by Professor Christoph Kerzig of Johannes Gutenberg University Mainz has introduced an innovative method that simplifies the synthesis of high-efficiency dyad photocatalysts. Their approach strategically utilizes two commercially available salts to form ionic pairs through electrostatic interactions—similar to how sodium and chloride ions form table salt. This synergy between the ions not only simplifies the synthesis process but also enhances the photocatalytic performance. Lead author Matthias Schmitz emphasizes the accessibility of this method, which comes at a fraction of the cost compared to the traditional synthesis of catalytic materials.
The research team undertook rigorous experimentation, utilizing advanced spectroscopy techniques to optimize the identified Coulombic dyads. Their investigations, powered by large-scale laser setups, aimed to elucidate critical reaction steps—ranging from light absorption to the activation of molecules. Among their findings, amounts of photoactive materials could be significantly reduced while still maintaining effective catalytic reactions, representing a considerable improvement in efficiency. Initial test reactions demonstrated the effectiveness of the new catalysts, achieving goals like forming new carbon-carbon bonds and the photooxygenation of wood-derived substrates, with results indicating a pronounced advantage over conventional catalysts.
Indeed, the research uncovered that the choice of solvent significantly influences the efficiency of the photocatalytic reactions. The concept of a “toolbox” approach allows for the design and combination of different photoactive anions and cations, adhering to specific solvent conditions to optimize reaction outcomes. These findings pave the way not only for enhancing current photocatalytic technologies but also for developing a general strategy to improve light-driven reactions on a broader industrial scale. The implications of such advancements could lead to significant strides in sustainable chemical production and environmental processing.
The revelations from Professor Kerzig’s team represent more than just a scientific milestone; they encapsulate the spirit of innovation in chemistry. With the potential for scaled-up production and enhanced efficiency, the concept of Coulombic dyads could redefine the landscape of photocatalysis. By lowering costs and streamlining production processes, this research may lead the way to more sustainable practices in various industries, demonstrating the undeniable synergy between scientific advancement and environmental stewardship. As researchers continue to refine these methods, the outlook for photocatalytic technologies appears promising, ushering in an age of efficiency and accessibility in chemical synthesis.

Leave a Reply