The significance of rare-earth metals in modern technology cannot be overstated. These metals, primarily consisting of lanthanides, are essential in numerous applications, including electronics, green energy solutions, medical imaging, and national defense systems. Despite their name suggesting scarcity, many of these elements are actually quite abundant in nature. However, the real challenge lies in their extraction and purification. The process to isolate individual lanthanides is complex due to their remarkable similarity in physical and chemical properties, making their separation a complicated endeavor. A recent breakthrough by researchers at Oak Ridge National Laboratory (ORNL) aims to tackle this challenge head-on with the discovery of a novel ligand, often referred to as a “chemical chameleon.”
The isolation of specific lanthanides from a mixture is a process fraught with difficulty. The ions of these metals are exceedingly similar in size and charge, leading to significant challenges in chemical separation techniques. Traditional methods of extraction involve the use of ligands—chemical agents that preferentially bind to certain elements within a mixture. These ligands are essential for effective separation but often operate under strict conditions that facilitate only a linear extraction sequence, either from heavy to light or vice versa. This not only increases the time and cost of the extraction process, but it also generates considerable waste, raising environmental concerns.
Scientists have made strides in refining these separation techniques; however, the quest for a more efficient method continues. The ligands used in the current systems display fixed preferences for separating certain lanthanides, resulting in labor-intensive and resource-heavy processes that are not always sustainable or practical from an environmental viewpoint.
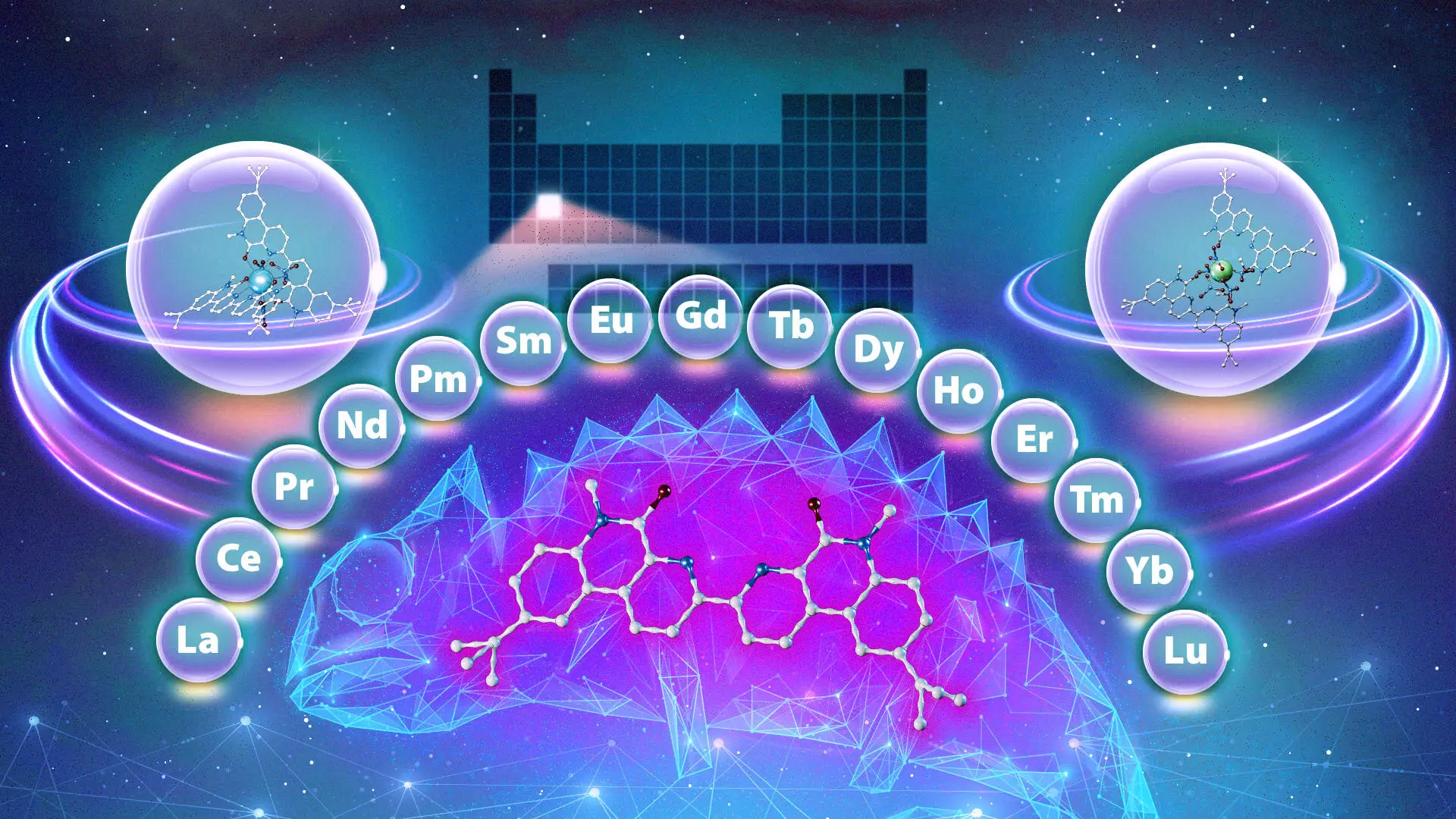
In a compelling turn of events, the research team at ORNL in collaboration with Vanderbilt University has identified a unique ligand that exhibits remarkable versatility akin to a chameleon. Unlike typical ligands that exhibit a rigid selective affinity towards either heavier or lighter lanthanides, this new chameleon ligand changes its binding properties based on the experimental conditions, particularly the acidity of the solution and the interaction time. This adaptability allows it to bind to different lanthanides effectively, significantly enhancing the potential for streamlined purification processes.
The ability of this ligand to separate lanthanides in any desired order poses a significant breakthrough. It can adapt its behavior to preferentially extract the heaviest, lightest, or mid-weight lanthanides, depending on the specific conditions. This adaptability not only offers a more efficient approach to rare-earth metal extraction but also significantly reduces the number of steps traditionally required in this process.
The implications of this discovery extend beyond the laboratory. If commercialized, the chameleon ligand could revolutionize the entire landscape of rare-earth metal purification. The chemical industry could witness not only a reduction in operational costs but also a decreased environmental footprint due to the minimized waste generated in the extraction process. By utilizing a single compound for multiple separations, the industry could transition to a more sustainable model of resource extraction.
Furthermore, this breakthrough opens the door for further research into similar compounds that could exhibit chameleon-like properties. As scientists delve deeper into understanding the mechanisms behind the ligand’s unique behavior, the potential for discovering and developing new ligands may expand significantly, leading to even more efficient methods for rare-earth metal extraction.
While the current findings represent a significant milestone, the journey does not end here. The research led by Subhamay Pramanik and his colleagues intends to advance the study of the chameleon ligand to fully understand the intricate mechanisms at play. This deeper understanding may pave the way for creating an array of ligands that can be tailored to specific industrial needs, leading to a new era in chemistries for metal extraction and purification.
The discovery of this unique chameleon ligand could redefine how rare-earth metals are extracted and purified. With its versatile binding behavior, this ligand promises a more efficient, sustainable, and economically viable approach to accessing the crucial materials needed for a variety of modern technologies, from renewable energy systems to advanced medical devices. As research continues, the potential for future advancements in this field appears promising, poised to address some of the pressing challenges facing the rare-earth metal industry today.

Leave a Reply