In recent years, carbon dioxide (CO2) emissions have emerged as a critical driver of global warming and climate change. As industries continue to expand and human activity intensifies, the release of CO2 into the atmosphere reaches alarming levels, raising concerns about the future of our planet. Among the potential strategies for mitigating these emissions, the use of cement-based materials has garnered considerable attention. Cement plays a vital role in construction, yet it is also a significant contributor to CO2 emissions. The ongoing research into carbonation processes presents a viable approach to capture CO2 and sequester it as stable carbonate minerals, thereby addressing some of the pressing challenges associated with climate change.
At its core, the carbonation of cement-based materials involves a intricate series of chemical reactions that convert gaseous CO2 into solid carbonate forms. This process begins when CO2 dissolves in water, leading to the formation of carbonate ions (CO32-) in the presence of calcium silicate hydrates, the essential products of cement hydration. The carbonate ions subsequently interact with calcium ions (Ca2+), producing calcium carbonate precipitates. However, despite numerous studies and investigations exploring the intricacies of this reaction, a unified understanding of the underlying mechanisms remains elusive. The composition and stability of cement paste compounds introduce complexities that challenge researchers in their quest for clarity.
Factors Influencing Carbonation Efficacy
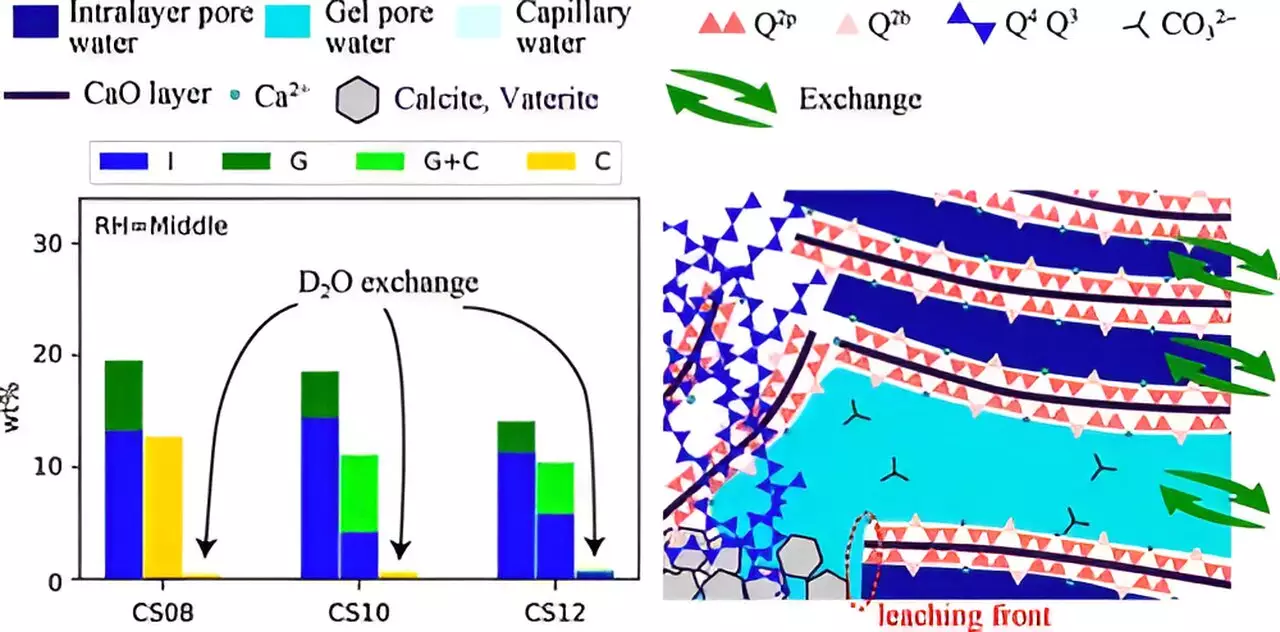
The efficiency of the carbonation process is influenced by numerous factors, including relative humidity (RH), the solubility of CO2, and the calcium/silicate (Ca/Si) ratio present within the calcium silicate hydrates. Water transport dynamics and ion interactions play critical roles in determining the effectiveness of CO2 absorption and mineralization. As researchers delve into the nano-scale pores found in C-S-H layers, the gel-pore water’s role becomes evident, raising further questions about how water movement contributes to carbonation outcomes.
A pivotal study led by Associate Professor Takahiro Ohkubo from Chiba University sought to illuminate these factors by investigating the carbonation dynamics under different RH conditions and varying Ca/Si ratios. This multi-institutional research team combined advanced techniques, including 29Si nuclear magnetic resonance and 1H NMR relaxometry, to evaluate water transport and the structural changes accompanying carbonation. By leveraging these innovative methods, the study aims to unravel the complex interactions that govern carbonation in cement-based materials.
Understanding natural carbonation, which occurs over extended periods, poses a considerable challenge in laboratory settings. To overcome this, the research team employed accelerated carbonation experiments, exposing synthesized C-S-H samples to CO2 concentrations significantly higher than those found in the environment. This approach allowed for a more efficient assessment of the carbonation mechanisms at play.
The samples were synthesized under controlled conditions, varying RH levels and Ca/Si ratios to simulate diverse environmental settings. By subjecting these samples to rigorous testing, the researchers sought to identify the precise structural changes induced by carbonation and the impact of these changes on pore characteristics. Their findings revealed that the structural integrity of the C-S-H network, including the collapse of its chain structure, is closely tied to both the Ca/Si ratio and RH conditions, ultimately influencing the efficiency of CO2 uptake.
The results of this investigation offer significant implications for the development of sustainable construction materials. By understanding the interplay of structural modifications and mass transfer during carbonation, researchers can design cement-based materials that effectively capture atmospheric CO2. The potential for creating new building materials that are carbon-negative could revolutionize the construction industry, contributing to global efforts to reduce carbon emissions.
Furthermore, the relevance of this research extends beyond construction; carbonation reactions are also prevalent in organic materials. Therefore, insights gained from this study may enhance our comprehension of natural carbonation processes in diverse environments, paving the way for innovative solutions to combat climate change.
This exploration of the carbonation mechanisms in cement-based materials highlights the ongoing efforts to devise effective strategies for CO2 reduction. By bridging the gap between fundamental research and practical applications, scientists like Associate Professor Ohkubo and his peers are making significant strides toward addressing one of the most pressing challenges of our time. As we continue to seek sustainable solutions, the findings from such studies serve as a beacon of hope, illustrating the potential of innovative materials to contribute to a healthier planet.

Leave a Reply