Aluminum oxide, often referred to as alumina, has long been recognized for its versatile applications across numerous scientific fields, from electronics to catalysis. The unique structural characteristics of aluminum oxide, especially its interface with other materials, have made it a subject of intensive research. However, the internal and surface atom arrangement within this material has been a longstanding enigma, an insulation feature complicating scientific inquiry and understanding of its properties.
Aluminum oxide (Al2O3) is not just an ordinary compound; it takes many forms, including crystals like sapphire and ruby. Its wide-ranging uses stem from its impressive insulating properties, but these very qualities have made it challenging for researchers to analyze the surface structure of alumina effectively. The internal arrangement of atoms in aluminum oxide is well-defined; however, discrepancies arise at the material’s surface, creating an intriguing contradiction that has baffled scientists for decades.
In 1997, the convoluted surface structure of aluminum oxide was highlighted as one of the “three mysteries of surface science”. Understanding the surface’s atomic arrangement is essential for exploring catalytic reactions within the material. These reactions could play a significant role in environmental and energy-related applications, which made resolving this structural mystery a high priority.
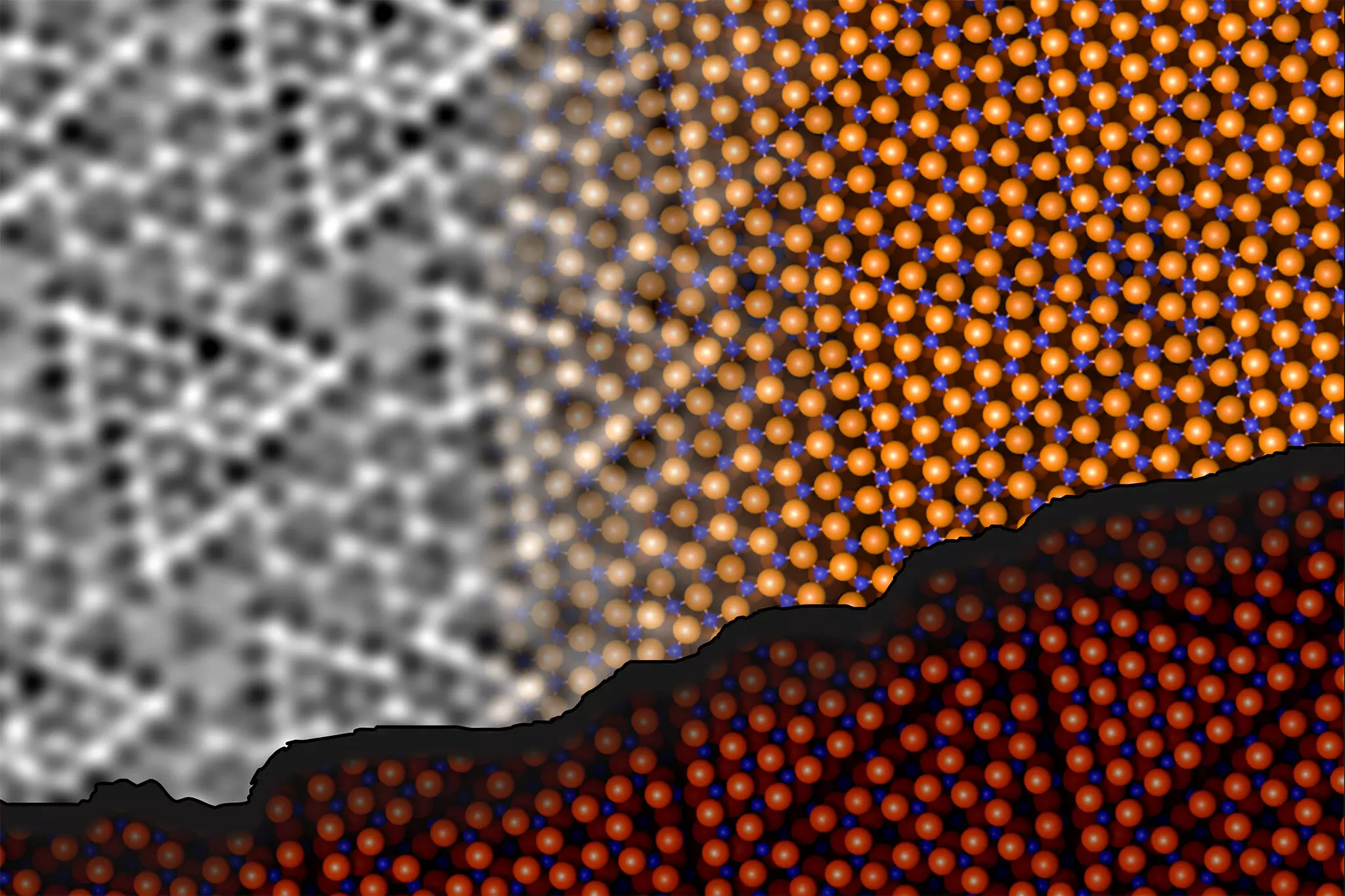
Recent advancements have paved the way for significant breakthroughs in illuminating the complex structure of aluminum oxide. Researchers at TU Wien and the University of Vienna have pioneered a method that blends experimental techniques with computational modeling to unravel this mystery. The team, composed of experts like Jan Balajka and Ulrike Diebold, employed noncontact atomic force microscopy (ncAFM) to meticulously map the aluminum oxide surface.
The ncAFM technique involves scanning a sharp tip over the material’s surface to detect variations in atom interactions indirectly. An innovative aspect of their approach was the use of an oxygen atom attached to the microscope tip, enabling the differentiation between aluminum and oxygen atoms on the surface. This method contrasts traditional atomic force microscopy, which typically requires physical contact that could alter the surface characteristics.
The researchers made compelling discoveries regarding how aluminum atoms interact with the oxygen atoms in the deeper crystal layers. It was observed that the aluminum atoms at the surface are not static but actively participate in forming chemical bonds with oxygen atoms located below. This atomic reconfiguration is crucial as it stabilizes the surface structure, contrary to previous assumptions about aluminum-oxygen ratios.
By mapping out this intricate dance between surface and subsurface atoms, the team demonstrated that instead of varying in atomic ratios, the layers maintain a constant aluminum-to-oxygen ratio while rearranging atomically to minimize energy and maximize stability.
The complexity of aluminum oxide’s structural configuration necessitated the incorporation of advanced computational algorithms, particularly machine learning, to refine their findings. The researchers utilized state-of-the-art algorithms in tandem with classical computational methods, generating a comprehensive three-dimensional model of the surface. This cutting-edge approach enabled them to explore the myriad atomic arrangements that would align with experimental results, leading to a more profound understanding of the material’s structural parameters.
Andrea Conti, a key contributor to the computational modeling efforts, noted that the collaboration between experimental and computational research was crucial. It highlighted the synergy between theoretical insights and practical applications, enabling breakthroughs not only in understanding aluminum oxide but also creating a framework applicable to various materials in the field of material science.
The unraveling of the aluminum oxide surface structure heralds notable advancements across disciplines, from catalysis to nanotechnology. With a clearer understanding of its chemical interactions at the atomic level, researchers can now develop new strategies to optimize aluminum oxide for specific applications, potentially enhancing its performance as a catalyst in environmental applications or in the creation of new electronic components.
The innovative combination of ncAFM and machine learning has significantly advanced the understanding of aluminum oxide’s surface structure. As researchers continue to explore these findings, they carry the potential to impact various technological fields, offering a promising frontier in material science with implications that reach far beyond the confines of aluminum oxide itself.

Leave a Reply