Recent research has unveiled a fascinating aspect of high entropy oxides—materials that exhibit considerable promise in the realm of electronic devices. As conveyed in a study published in the esteemed Journal of the American Chemical Society, it becomes clear that the synthesis methods employed play a crucial role in determining the material’s structural and functional properties. These oxides represent a new frontier in materials science due to their unique combination of elements, making them suitable for various applications including energy storage, catalysis, and electronic components.
High entropy oxides are characterized by their incorporation of five or more different transition metals within a single crystal structure. This unique amalgamation grants them remarkable electrochemical properties, which make them candidates for next-generation technologies. Alannah Hallas, a leading materials scientist from the University of British Columbia’s Blusson Quantum Matter Institute, emphasizes the flexibility and tunability of these materials. Such versatility is critical as it allows researchers and industrialists to optimize the properties of these oxides for specific applications by manipulating their synthesis conditions.
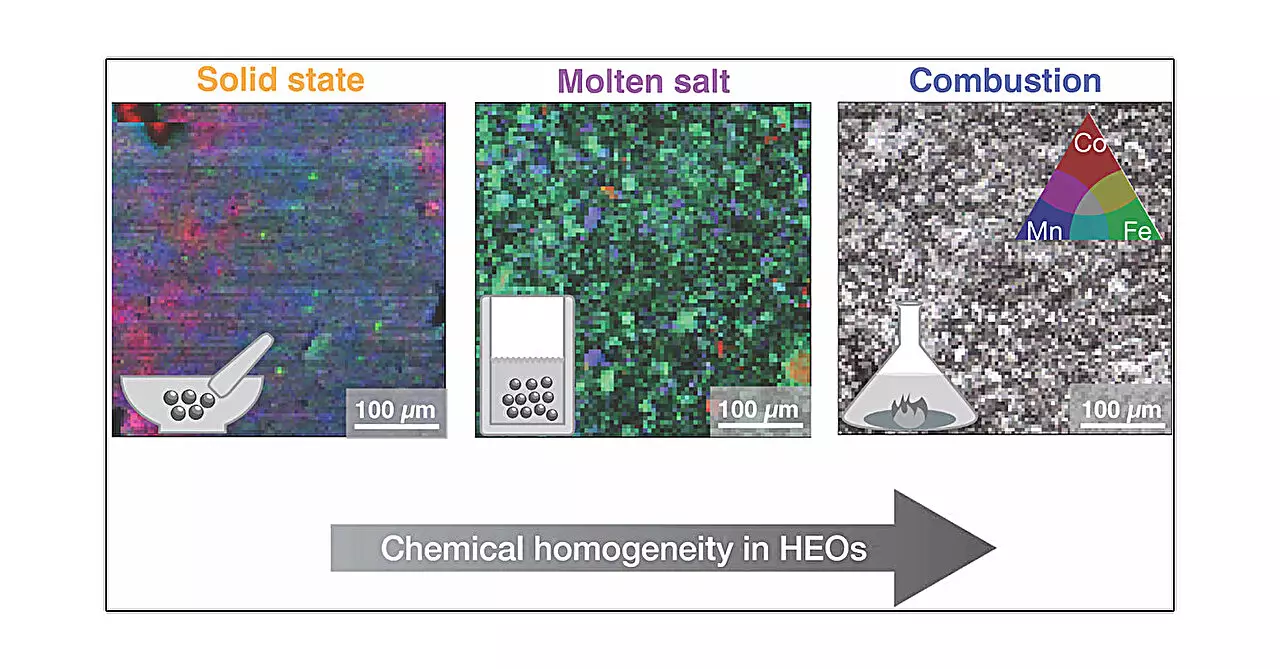
In the study, researchers explored five distinct synthesis methods: solid state, high pressure, hydrothermal, molten salt, and combustion synthesis, each representing a unique approach to material fabrication. These methods vary dramatically—ranging from traditional heating and cooling techniques to complex reactions that emulate natural geological processes.
The solid state method functions similarly to baking a cake—mixing metal oxides and subjecting the mixture to high temperatures to allow for bonding. In contrast, the high-pressure synthesis method introduces additional external pressure into the heating process, which significantly influences the arrangement of atoms within the material. Hydrothermal synthesis operates under conditions that replicate the formation of minerals, utilizing water and heat in a pressurized environment to facilitate crystal growth.
The molten salt method involves the use of liquid metal salts, creating a homogenous suspension that allows for controlled crystal precipitation upon cooling. Lastly, the combustion synthesis method diverges from the others by employing a gel-like precursor that ignites, producing materials rapidly through a combustion reaction. Each of these methods generates materials that, while sharing the same basic structure, can possess radically different microstructures and local arrangements of atoms.
A pivotal finding of this research is the profound effect synthesis methods impose on the local structures of high entropy oxides. While the overall average structure of the materials remained consistent, subtle variations in microstructure emerged. For instance, combustion synthesis yielded the most homogeneous samples, thus enhancing the potential performance of materials created through that method.
Lead author Mario Ulises González-Rivas pointed out that the variations in local and microstructural characteristics could critically influence how these materials perform in various applications, particularly concerning energy systems. This insight could lead to a new paradigm in material design, where researchers strategically choose synthesis methods based on desired structural characteristics.
The implications of this study extend far beyond the confines of academic research. As high entropy oxides burgeon into a key component of advanced energy solutions, understanding the impact of synthesis techniques will be vital for industry stakeholders looking to implement these materials effectively. González-Rivas notes that this study opens up a new avenue for optimization in applied settings, enabling engineers and scientists to design better energy systems.
Collaboration between diverse research institutions, such as the University of British Columbia and the Max Planck Institute for Solid State Research, underscores the global interest in optimizing high entropy oxides. This cooperation is essential for pushing forward the boundaries of materials research and addressing pressing energy challenges through innovative technologies.
High entropy oxides represent a promising class of materials influenced significantly by their synthesis methods. As research continues to explore the vast possibilities these materials hold, a focus on tailored synthesis could lead to breakthroughs in energy applications and electronic devices—ultimately shaping our technological landscape for years to come.

Leave a Reply