In a groundbreaking study published in *Nature Communications*, a team of researchers led by Samuel Schwab at the Leiden Institute of Chemistry has reshaped our understanding of histones in single-celled organisms such as bacteria and archaea. For ages, the academic consensus suggested that histones—proteins crucial for DNA structure—were exclusive to more complex organisms. However, recent investigations have unveiled that these proteins are not only present in single-celled organisms but exhibit an astonishing level of diversity, with Schwab’s research identifying no fewer than 17 distinct groups.
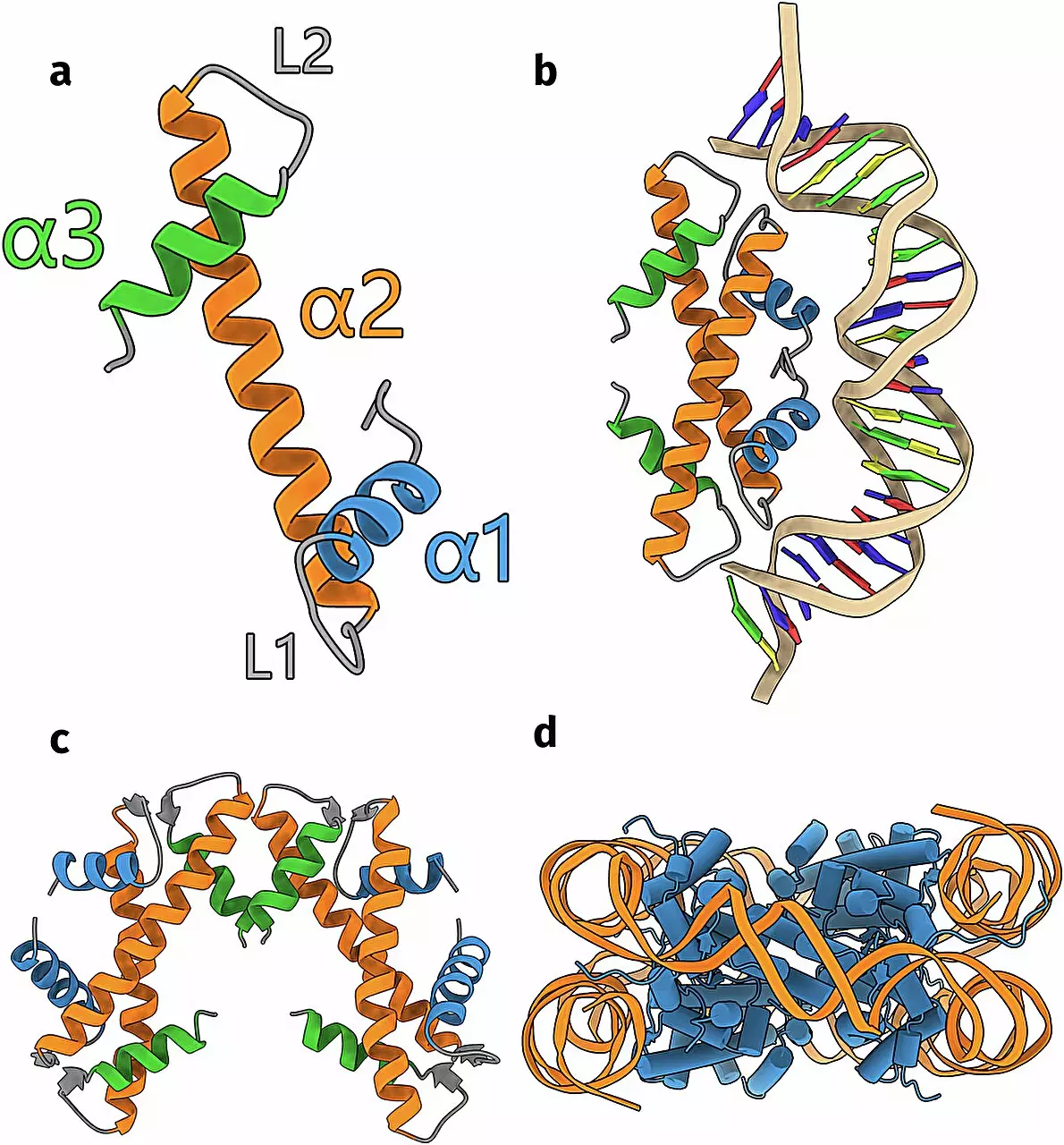
Histones serve a fundamental role in the compaction of DNA, a molecule that would otherwise be too unwieldy to fit within the confines of a cell. They do this by forming nucleosomes, structures around which DNA is coiled. This study builds on a previously established understanding but propels the conversation forward by challenging prior assumptions and expanding our insight into the genetic world of simpler life forms.
Integral to this investigation was the use of a pioneering artificial intelligence algorithm known as AlphaFold. This innovative tool effectively predicts protein structures from specific DNA sequences, a task that previously required extensive lab work and resources. By harnessing AlphaFold and utilizing the Leiden supercomputer facility ALICE, Schwab sifted through a stunning repository of 6,000 DNA sequences, searching for cryptic genetic instructions that could lead to the discovery of previously unknown histones.
The implications of this technological application extend beyond mere efficiency; they represent a significant leap in our ability to predict biological structures. Not only did Schwab’s team successfully validate their AI-driven predictions with experimental results, but they also established a framework for future analyses. This marriage of biology and cutting-edge technology demonstrates the potential for interdisciplinary collaboration to propel scientific discovery.
Among the 17 identified groups of histones are those with established functions as well as entirely novel structures, raising fundamental questions about the roles these proteins may play in genetic regulation. Notably, through experimental validation, Schwab’s team confirmed that one newly identified histone exhibited structural characteristics that aligned closely with AlphaFold’s predictions.
The functional ramifications of this diversity are profound. Schwab notes that the histones can exhibit various forms of interaction with DNA, encompassing not just the familiar nucleosomal wrapping but also more intricate configurations, such as loop formations connecting distinct DNA segments. This complexity suggests that histones have evolved specialized roles that contribute to the genetic sophistication of archaea and bacteria, further underscoring the need for a reevaluation of their cellular functions.
A particularly intriguing aspect of this research involved the observation that some histones appeared to bind not to DNA itself, but rather to cellular membranes. This finding posits the existence of functions beyond mere DNA organization, hinting at a multifaceted role for histones that encompasses broader cellular interactions. These revelations open a doorway to new lines of investigation that could deepen our understanding of cellular processes, potentially rewriting textbooks on cellular biology.
As Schwab aptly summarizes, the insights garnered from this study represent just the tip of the iceberg. There remains a vast expanse of knowledge to be uncovered regarding the precise roles of these diverse histones. The implications of this research stretch far beyond academia, potentially influencing applications in biotechnology and medicine, where understanding DNA management is crucial.
The work of Schwab and his colleagues stands as a pivotal contribution to the field of genetics, especially concerning organisms that have long been underappreciated in terms of their biological complexity. As we delve deeper into the structure and function of histones within single-celled organisms, we discover not only a more intricate view of cellular life but also contemplate how these organisms may inform our understanding of evolution and the fundamental principles governing all life forms. The journey into this uncharted territory is just beginning, and there is still much to learn from the humble yet capable histones that organize and manage our DNA. As research in this area continues to evolve, so too will our grasp of biological mechanisms, potentially unraveling secrets that lie at the very heart of life itself.

Leave a Reply