Cholesterol, often vilified in discussions of heart health, serves essential functions within the realm of cellular biology. A recent investigation led by Jason Hafner at Rice University has illuminated cholesterol’s intricate role in the architecture of cell membranes. This study, which has been published in the prestigious Journal of Physical Chemistry, seeks to demystify how cholesterol interacts with cell membranes and their receptors, possibly heralding breakthroughs in the understanding of several diseases, including cancer.
The complexity of biomembranes—composed of lipids and proteins—poses significant challenges for researchers, particularly when it comes to understanding cholesterol’s placement and function within these structures. For years, the scientific community has grappled with elucidating cholesterol’s true nature within biomembranes. Hafner emphasizes the relevance of their findings, noting that understanding how cholesterol organizes within membranes could lead to significant advances in our comprehension of cancer and other diseases where membrane integrity is crucial.
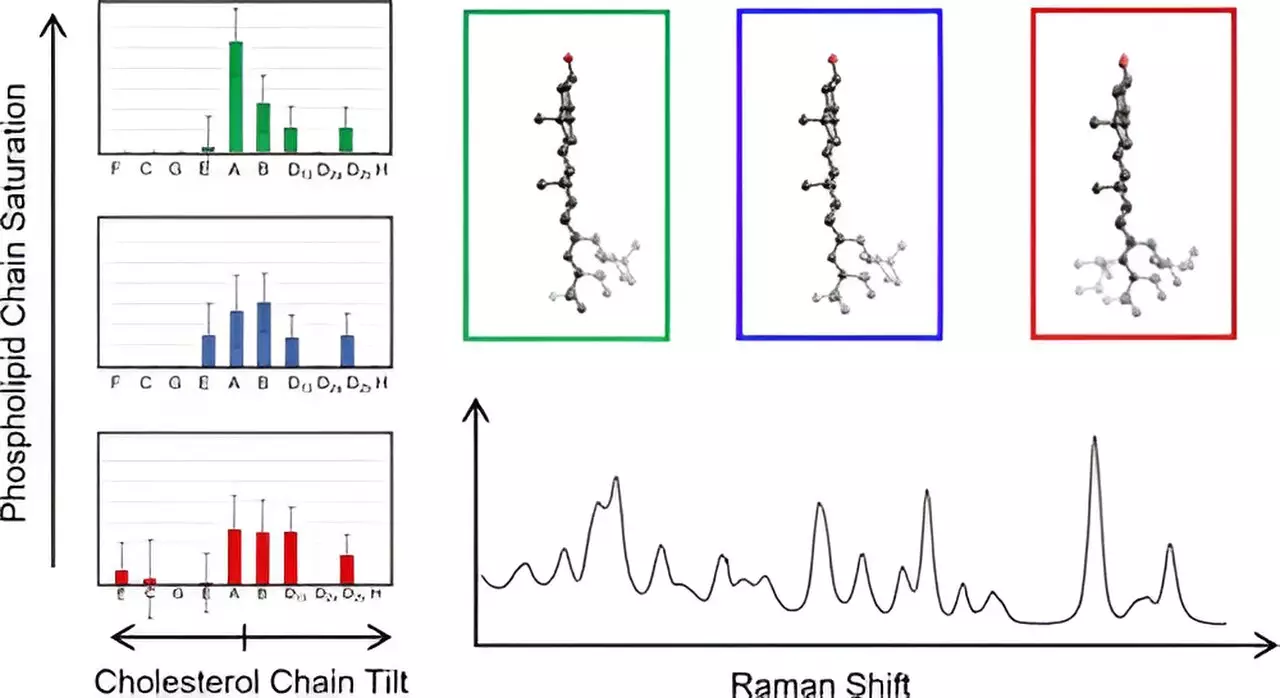
Innovative Techniques: The Role of Raman Spectroscopy
To unravel the mysteries of cholesterol’s behavior in membranes, Hafner’s lab employed Raman spectroscopy, an advanced analytical technique that generates detailed information about molecular vibrations through scattered laser light. This method was pivotal in examining the characteristics of cholesterol molecules embedded within complex membrane structures. By performing a detailed comparative analysis of the observed spectral data against theoretical models derived from density functional theory, Hafner’s team was able to gain unprecedented insights into the molecular structure and interactions of cholesterol.
One remarkable outcome of this research was the ability to categorize cholesterol molecules based on their structural properties. The team computed Raman spectra for a variety of cholesterol structures and made a surprising discovery: cholesterol molecules within the same structural group displayed identical low-frequency spectral characteristics. This remarkable uniformity significantly simplifies the analytical process, enabling researchers to map out the chain structures of cholesterol within natural membrane environments effectively.
This groundbreaking achievement also sheds light on previously unrecognized variations in cholesterol structures. By clarifying the relationship between the position of cholesterol chains relative to the membrane’s planar rings, Hafner’s findings establish a new foundation for future studies on membrane organization and its relation to cellular functions.
The implications of Hafner’s research are vast, signaling a transformative shift in our understanding of cholesterol’s role within biomembranes. With the methodology established in this study, researchers are now better equipped to explore the biochemical underpinnings of diseases linked to membrane abnormalities. The collaborative efforts of Hafner’s team, including notable students and researchers, set the stage for future investigations that may uncover therapeutic targets or even new treatment strategies for diseases like cancer, where cell membrane integrity is paramount. As the scientific community continues to peel back the layers of complex biomolecular interactions, studies like this provide a crucial building block for advancing our understanding of cellular health and disease.

Leave a Reply