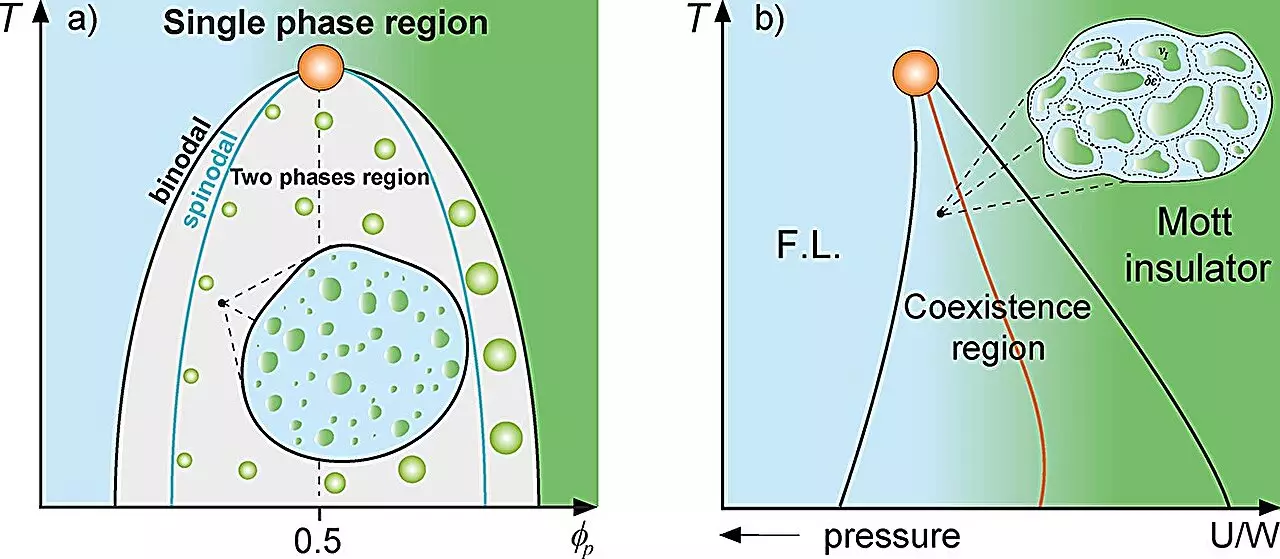
In the realm of physics, the conceptual framework used to analyze mixtures of different substances offers important insights that transcend traditional boundaries. Classical mixture theory serves as a lens through which scientists can explore the complex interactions that occur within multi-component systems. A vivid example is the behavior of supercooled water, where both high- and low-density phases co-exist, or the behavior of metals within insulators that characterizes the Mott metal-insulator transition. At the forefront of applying this theoretical framework to biological systems is research emerging from São Paulo State University (UNESP) in Brazil, where a team led by Professor Mariano de Souza has pursued a groundbreaking investigation into the phenomenon of protein compartmentalization within cells.
The research published in the journal Heliyon introduces an innovative analogy between protein droplets in biological systems and the well-documented Griffiths phase in magnetism. In magnetic systems, such as those involving ferromagnetic or paramagnetic materials, “rare regions” appear, resulting in significant alterations in dynamic behavior. Similarly, the study posits that protein droplets within cellular environments can be categorized as these ‘rare regions,’ particularly in the context of liquid-liquid phase separation. According to lead author Lucas Squillante, these insights offer a fresh perspective on longstanding questions regarding how cellular dynamics are maintained in the face of myriad interactions among biological macromolecules.
Professor de Souza and his team employed diverse thermodynamic concepts to explain how varying protein concentrations lead to phase separation—the process wherein proteins condense into distinct droplets that are critical for cellular organization. By utilizing models such as the Grüneisen parameter, the Flory-Huggins model, and the Avramov-Casalini model, they elucidate the mechanisms behind these dynamic reductions in cellular contexts, bringing to light the Griffiths-like cellular phase’s relevance to understanding life’s origins.
The Nexus Between Dynamics and Life’s Evolution
Intriguingly, the research also draws connections to the origins of life itself. It aligns with the theories proposed by early biologists like Aleksandr Oparin, who hypothesized about the conditions necessary for primordial molecules to evolve into more complex entities. The team’s findings suggest that protein coacervates, formed through liquid-liquid phase separation, may have played a critical role in this evolutionary journey. The connection is further emphasized by the notion of homochirality, which refers to the predominance of one form of chirality within organic molecules—a characteristic essential for biological function.
This perspective is crucial because it not only highlights how certain conditions facilitated the emergence of life but also helps explain the selection processes that underlie the evolution of cellular structures capable of maintaining those dynamics. The overarching theme remains consistent: the manner in which proteins behave within cellular compartments can significantly influence gene expression and evolutionary stability over time.
The broader implications of the Griffiths-like cellular phase extend into the medical realm, particularly concerning disease understanding and treatment. Research co-authored by Professor Marcos Minicucci illustrates how liquid-liquid phase separation can influence diseases such as cancer, neurodegenerative disorders, and even COVID-19. For instance, in cancer, proteins associated with tumorigenesis may become compartmentalized in ways that alter their roles and interactions. This potentially opens avenues for new therapeutic strategies that consider protein droplet formation as a critical factor in disease management.
Moreover, specific diseases such as cataracts and neurodegenerative conditions demonstrate the complex interplay between protein phase separation and cellular health. The emergent nature of these droplet formations has been implicated in various pathologies, indicating that understanding this cellular phase could lead to transformative insights in treating multifaceted health issues.
As the research from UNESP demonstrates, the intersection of physics, biology, and medicine can yield profound insights into fundamental phenomena. The work of de Souza’s group underscores the importance of interdisciplinary collaboration in enriching our comprehension of the complex systems that govern life. By harnessing principles from condensed matter physics to investigate the intricacies of cellular dynamics, the team paves the way for new research directions that hold promise not only for understanding the mechanisms of life but also for devising innovative approaches to health and disease management. In this exciting frontier of scientific inquiry, the Griffiths-like cellular phase emerges as a key component in unraveling the enigma of life and, perhaps, the very essence of existence as we know it.

Leave a Reply