The conversion of carbon dioxide (CO2) into usable chemical products represents a vital avenue for addressing climate change and promoting sustainability. Among various methodologies, the electrochemical reduction of CO2 stands out as a potentially transformative process. This approach allows for the transformation of a greenhouse gas into valuable resources while simultaneously addressing excess CO2 emissions. However, the intricate dynamics of selectivity in these reactions have largely centered on catalyst development and optimization, often sidelining the significant influence electrolyte composition can exert on the outcome of CO2 reduction processes.
Recent advancements have started to challenge this oversight, as illustrated by a study presented in *Angewandte Chemie International Edition*. This research introduces a metal-organic framework (MOF) electrocatalyst, known as FICN-8, which exemplifies how differences in electrolyte constituents can dramatically alter the selectivity of CO2 reduction products. Led by esteemed scientists Prof. Cao Rong and Prof. Zhang Teng from the Fujian Institute of Research on the Structure of Matter, this investigation underscores a paradigm shift that recognizes electrolytes not merely as passive solvents but as active participants in electrochemical reactions.
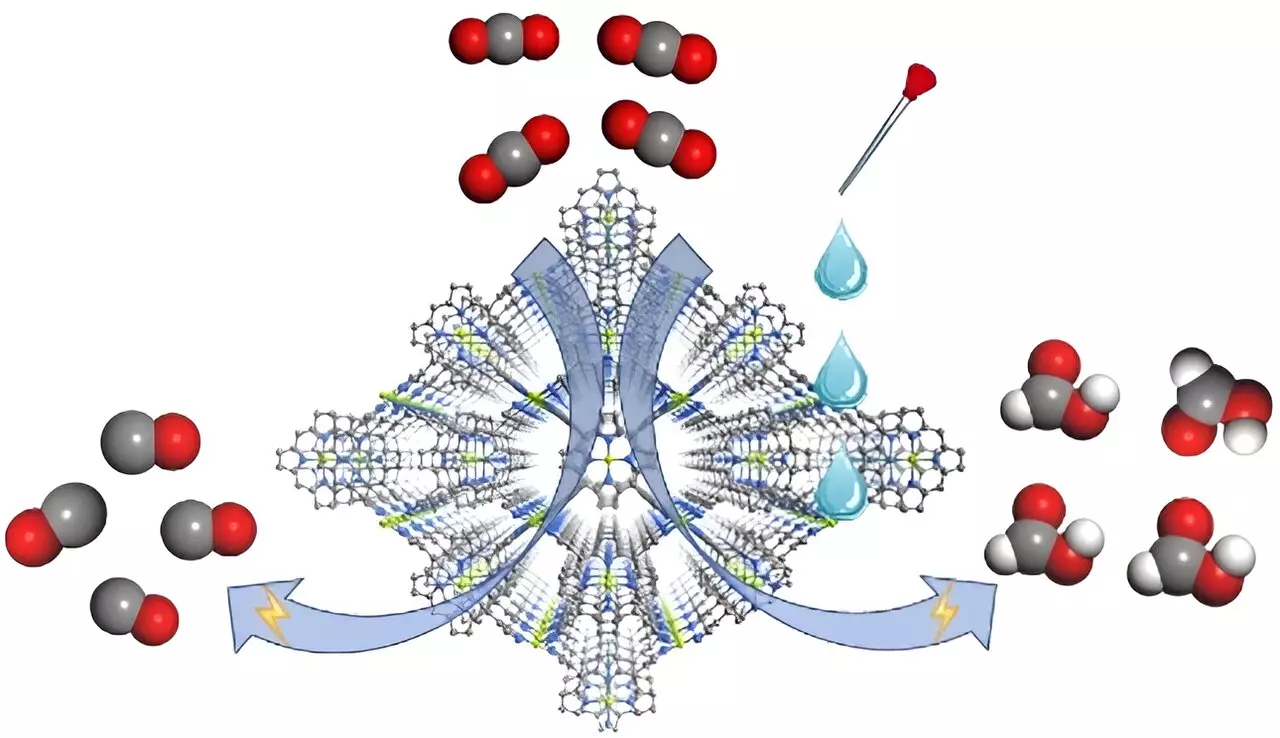
FICN-8 is constructed utilizing copper-derived ligands integrated with pyrazolate building blocks, creating a 3D porous architecture. This structural design enhances the accessibility of the catalytic sites, promoting efficient reaction pathways. As a heterogeneous electrocatalyst, FICN-8 allows for tuning the solvent and electrolyte composition across diverse conditions, bridging gaps in presently existing methodologies. In practice, the research indicated that in an electrolyte of tetrabutylammonium hexafluorophosphate (TBAPF6) combined with acetonitrile, FICN-8 achieved remarkable selectivity with CO as the primary product, reaching an impressive 95% selectivity.
The study’s pivotal insight emerges when water or trifluoroethanol (TFE) is introduced into the electrolyte as a source of protons. This seemingly simple alteration led to a significant shift in product distribution from CO to formic acid, with the latter achieving a peak Faradaic efficiency of 48% in the presence of water at a concentration of 2.65 mol/L or 0.55 mol/L TFE. This phenomenon raises important questions about how electrolytic environment adjustments can steer the pathways of electrochemical transformations, a perspective relatively unexplored until now.
To understand the underlying mechanisms governing these selectivity changes, the researchers employed kinetic isotope effect (KIE) measurements which indicated distinct reaction pathways. While CO production showed minimal isotope-dependent variation, the formation of formic acid exhibited a significant KIE value of 3.7 ± 0.7, signifying the essential role of protons in its synthesis. Theoretical models further highlighted the importance of hydride adsorption on the catalyst’s porphyrin N site as a pivotal step in forming formic acid, delineating a separate pathway for CO generation that operates independently of proton concentrations.
The implications of this research extend beyond immediate practical applications, suggesting a broader principle concerning the interactions between catalysts and electrolytes in electrochemical processes. By acknowledging the electrolyte’s role, researchers can envisage new frameworks for constructing tailored catalyst-electrolyte systems aimed at maximizing the production of high-value chemicals from CO2. Such strategies are essential as we move toward sustainable chemical transformations and strive to mitigate climate change through innovative scientific development.

Leave a Reply