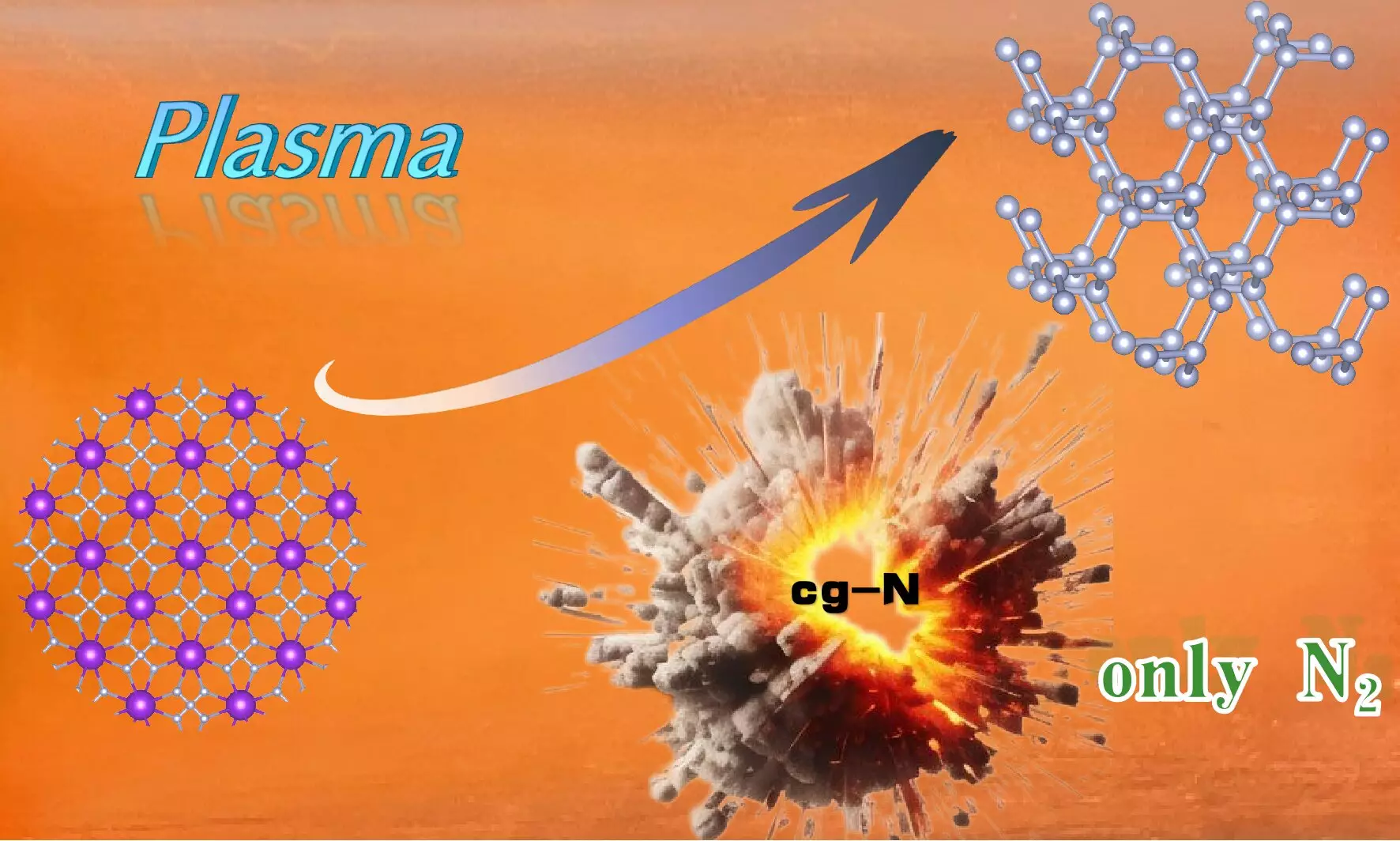
Recent advancements in the field of materials science have paved the way for the exploration of high-energy-density materials, which hold significant promise for applications ranging from propulsion systems to energy storage. Among these innovative materials, cubic gauche nitrogen (cg-N) has emerged as a noteworthy contender due to its exceptional energy characteristics. Researchers led by Prof. Wang Xianlong at the Hefei Institutes of Physical Science, part of the Chinese Academy of Sciences, have successfully developed a method for synthesizing cg-N at atmospheric pressure, thereby eliminating some of the challenges posed by traditional synthesis methods.
Cg-N is distinguished by its unique structural configuration, where nitrogen atoms are bonded through N-N single bonds, producing an arrangement tantamount to that of diamond. This structural similarity underpins its high energy density, making it an attractive option for future applications in the energy sector. Notably, cg-N decomposes solely into nitrogen gas, which indicates its potential for environmentally friendly applications, especially in energy generation and propellant technologies.
The synthesis of cg-N at atmospheric pressure has been a critical focus, as high pressure can complicate practical applications. The research team leveraged the plasma-enhanced chemical vapor deposition (PECVD) technique to develop a method that circumvents many traditional hurdles associated with material synthesis. By using potassium azide (KN3) as a precursor—chosen for its relatively low toxicity and less explosive nature—the researchers benefitted from the strong electron transfer capabilities inherent in potassium. This choice of precursor significantly contributes to the safety and efficacy of the synthesis process.
Stability and Computational Guidance
A significant aspect of this research is the application of first-principles calculations, which served as a foundational tool for predicting the stability of the cg-N material under diverse conditions. Prior studies indicated that surface instability could lead to the premature decomposition of cg-N, particularly at lower pressures. To mitigate this risk, the research team proposed a dual approach: stabilizing surface suspension bonds and manipulating charge transfer interactions. Their findings indicated that these adjustments could prolong the stability of cg-N even at elevated temperatures, up to 750 K under atmospheric conditions.
Thermal Characteristics and Future Implications
Thermogravimetric-differential scanning calorimetry (TG-DSC) assessments confirmed that synthesized cg-N retains thermal stability up to 760 K, after which rapid thermal decomposition occurs. This revelation not only underscores the robustness of the synthesized material but also provides critical insights into its behavior under operational conditions. As the study presents a reliable method for producing high-energy-density materials, it opens avenues for further research and development in creating innovative materials for various applications, including aerospace and beyond.
The work led by Prof. Wang Xianlong represents a significant milestone in the synthesis of high-energy-density materials. By focusing on the atmospheric-pressure synthesis of cubic gauche nitrogen, the research team has not only demonstrated the feasibility of producing such advanced materials but also broadened the scope for future exploration of nitrogen-based materials. This breakthrough could be instrumental in developing safer, more efficient materials tailored for numerous high-energy applications, potentially transforming sectors reliant on energy-dense compounds.

Leave a Reply