Researchers at the National University of Singapore (NUS) have made noteworthy advancements in the field of organic chemistry, particularly in the sustainable production of trisubstituted Z-alkenes. These compounds are critical components in various biologically active molecules and are essential for stereospecific reactions that lead to the formation of sp3-hybridized structures. Typically, the synthesis of these alkenes has encountered substantial obstacles, primarily due to the energetic instability of the Z-isomers compared to their E-isomer counterparts. However, the innovative method developed by the NUS team presents a significant leap forward in addressing this challenge.
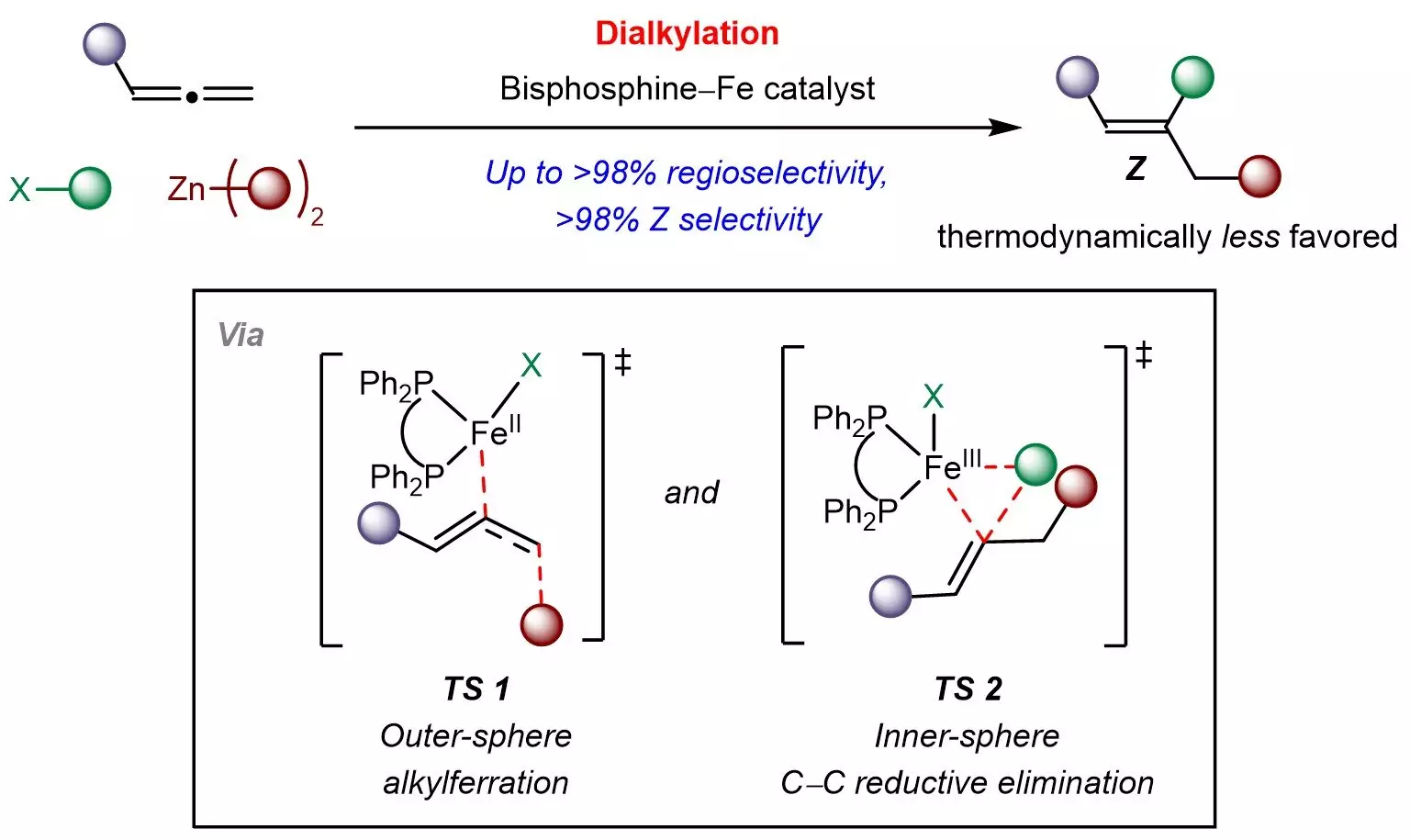
The NUS research team, led by Associate Professor Koh Ming Joo, has devised a pioneering approach using a cost-effective and widely available bisphosphine–iron catalyst. This catalyst facilitates an integration process where allenes interact with basic chemical entities such as sp3-hybridized organohalides and organozinc reagents. The beauty of this multicomponent framework lies in its capacity to manipulate both the site of the reaction and the desired Z-selectivity. The implication of using iron—an abundant, non-toxic transition metal—not only enhances the economic efficiency of the method but also aligns with the growing demand for environmentally friendly practices in chemical synthesis.
The practical application of this newly minted technique is illustrated through its role in simplifying the preparation of a specific glucosylceramide synthase inhibitor, a compound where the Z-configuration is pivotal for its biological activity. This notable application not only demonstrates the functional utility of the research but also amplifies its relevance in drug discovery and other medicinal chemistry arenas. Moreover, the collaboration with experts from The Chinese University of Hong Kong and the Agency for Science, Technology and Research (A*STAR) marks a critical nexus of interdisciplinary efforts driving innovation in this domain.
The researchers have proposed a distinctive mechanism underpinning their process, characterized by outer-sphere radical-mediated alkylferration followed by inner-sphere carbon-carbon bond formation. This offers vital insights that could aid in the design of kinetically controlled reactions not only for allenes but for a broader range of π-systems. Furthermore, the team is actively pursuing the development of additional multicomponent transformations aimed at converting readily available raw materials into value-added chemical products, thereby paving the way for advancements in industrial applications.
The work by NUS chemists marks a crucial step in the synthesis of trisubstituted Z-alkenes, providing a robust method that addresses long-standing challenges in the field. With implications that extend beyond mere academic curiosity into practical applications in pharmaceuticals and industrial chemistry, this research not only fills existing knowledge gaps but also opens avenues for further exploration and innovation. As these researchers continue to refine their methodologies and broaden their scope, the potential for impactful scientific contributions remains significant.

Leave a Reply