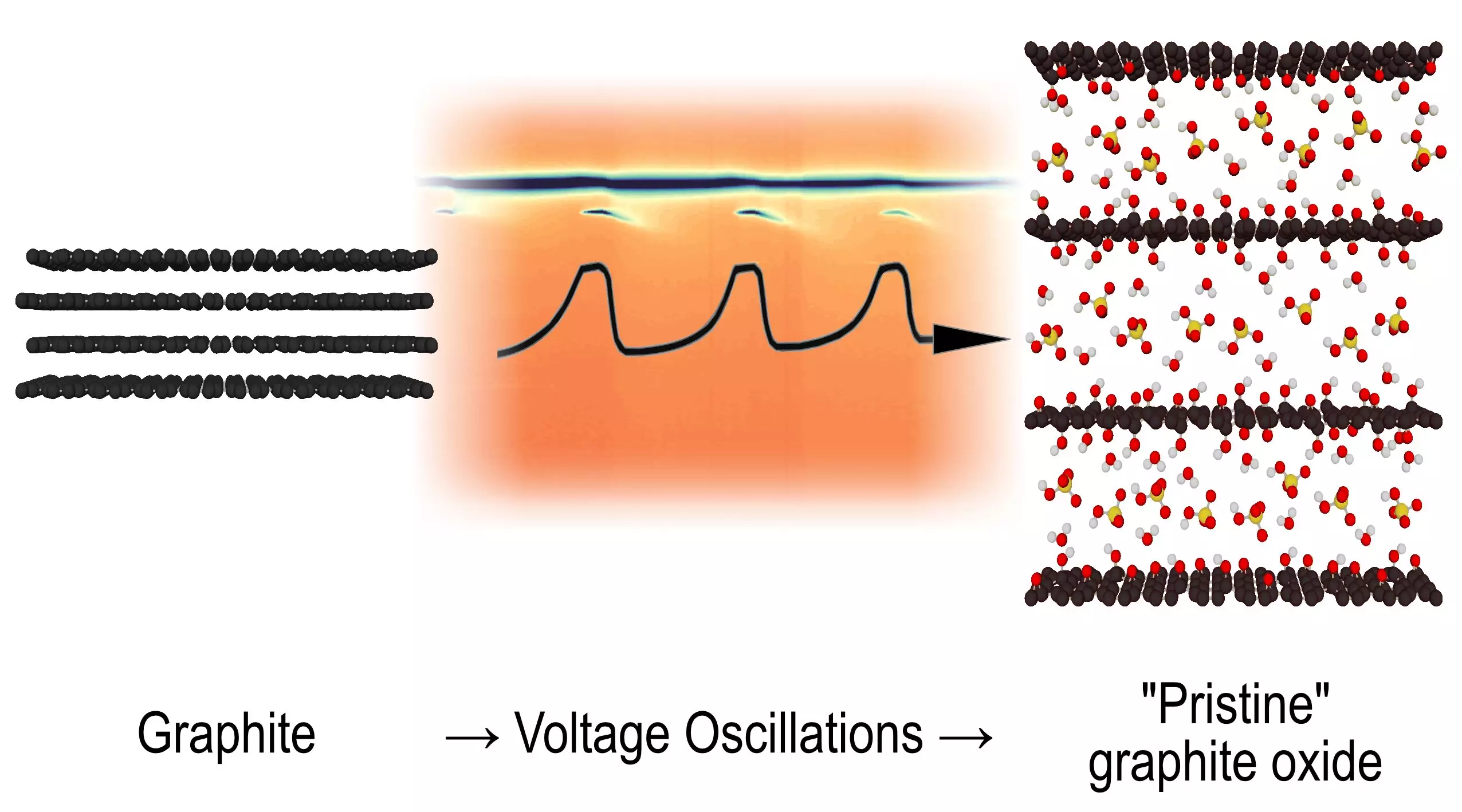
For half a century, the peculiar reactions involving graphite oxidation remained an intriguing mystery within the scientific community. Researchers at Umeå University have finally shed light on this decade-long enigma, revealing an unexpected dimension of chemical reactivity. This recent study unveils the transformation of graphite into graphite oxide during electrochemical oxidation, significantly enriching our understanding of oscillating reactions, a phenomenon recognized in both chemical processes and natural systems.
The oscillating reactions have garnered attention for their unique ability to exhibit periodic behavior, evident through visual transformation, often described metaphorically as a ‘dance’ between different states. In this study, the researchers successfully illustrated how specific structural changes occur within graphite oxide throughout this electrochemical reaction, charting uncharted territory in the study of chemical kinetics.
A pivotal factor enabling this discovery was the use of advanced synchrotron methods, which allowed the researchers to capture rapid X-ray diffraction scans in a matter of seconds. Unlike traditional methods, which could take considerable time and missed fleeting structural changes, the new technique enabled scientists to observe and document minute fluctuations in the material’s structure during the oxidation process.
What emerged from this research was not merely a confirmation of previous knowledge regarding graphite oxidation, but instead a dynamic observation of an intermediate phase that alternated existence throughout the reaction cycles. This phase appeared, vanished, and then reappeared cyclically, suggesting a novel type of oscillating reaction.
Professor Alexandr Talyzin, leading the research, highlighted the progression from a seemingly routine study of chemical reactions to a more profound understanding of chemical dynamics. “What began as a detailed study of a particular chemical reaction suddenly appeared to be a lot more interesting from the point of view of fundamental chemistry,” Talyzin explained. The study, detailed in the journal Angewandte Chemie, has profound implications for how scientists view oscillating reactions both in inorganic chemistry and broader chemical contexts.
First author Bartosz Gurzeda equally contributed to the study by producing a high-quality video that visually encapsulated the periodic changes occurring within the sample over several minutes, further emphasizing the transformative nature of the electrochemical process. Such visual documentation is invaluable for engaging broader audiences beyond the scientific community.
The discovery of this new oscillating reaction adds a significant chapter to the narrative of chemical kinetics and could bridge gaps in our existing theories and models. Historically, the notion of oscillating reactions was largely confined to living systems. This research challenges that paradigm, suggesting that such behaviors are equally applicable to inorganic chemistry, thereby expanding the frontier of what we consider possible in chemical interactions.
Furthermore, the implications of these findings resonate with the legacy of Ilya Prigogine, the Nobel laureate whose groundbreaking work in non-equilibrium thermodynamics changed the way we perceive chaotic systems. Prigogine’s research revealed that order could emerge from chaos, a theme echoed in the recent findings where the oscillations signify an underlying order within the transition of graphite to graphite oxide.
Looking ahead, researchers like Talyzin are optimistic that the discovery will spur the development of new theories aimed at fully articulating this unique form of oscillating reaction. “We hope that new theories will be developed to explain this new type of oscillating reaction, which may lead to the discovery of new similar examples,” Talyzin enthused.
The doorway opened by this discovery hints at the plethora of unexplored mechanisms in various chemical reactions, urging the scientific community to adopt a broader, more inclusive perspective on reaction dynamics. As we peel back the layers of chemical interactions, the age-old complexities of nature may gradually unveil themselves, fostering a deeper understanding that could transform both chemistry and our insights into the natural world.

Leave a Reply