As the world grapples with the escalating crisis of climate change, the need to transition from fossil fuels to sustainable energy sources has never been clearer. One promising avenue of research is the direct conversion of carbon dioxide (CO2) into useful products, particularly fuels. Among the groundbreaking developments in this field, a pioneering artificial photosynthesis system developed at the University of Michigan marks a significant shift in how we can utilize CO2 emissions. By efficiently binding carbon atoms together, this innovative technology not only provides a means for carbon reuse but also positions itself as a front-runner in producing essential hydrocarbon compounds.
At the heart of this research lies an advanced method of creating ethylene, a hydrocarbon pivotal in the production of plastics and other chemical products. Ethylene is recognized as the most abundantly produced organic compound globally, traditionally harvested through energy-intensive methods that involve high temperatures and gas emissions. The Michigan team’s new system, however, boasts an ability to produce ethylene with unprecedented efficiency and longevity, standing five to six times superior to what is generally reported in current artificial photosynthesis technologies. This remarkable achievement serves as a testament to the potential of harnessing solar energy effectively.
Professor Zetian Mi, a leader on this research project, remarked on how this system leverages sun-like light to initiate the breakdown of water and CO2, facilitating the chemical process necessary for ethylene synthesis. The implications are clear: by integrating this technology, industries could significantly reduce their CO2 emissions, thereby mitigating their environmental impact.
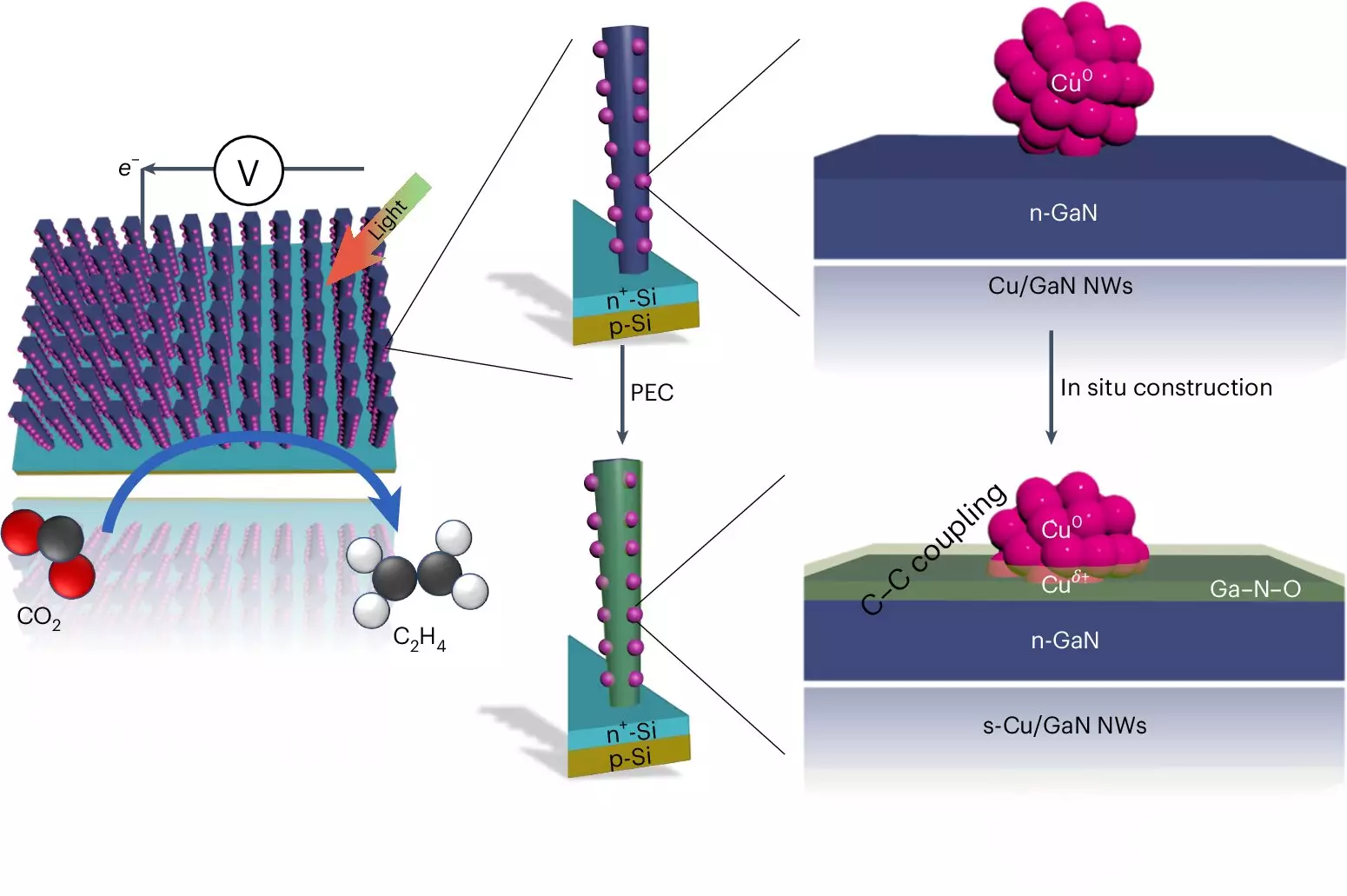
The operation of this innovative device involves several intricate components working in concert. Central to its function are gallium nitride nanowires, tiny structures that absorb sunlight to drive the conversion of CO2 and water. The reaction occurs at the copper clusters distributed across these nanowires, where the real magic happens. The absorption of light liberates electrons, resulting in the splitting of water molecules to produce hydrogen—a critical component for ethylene synthesis.
This entire process is a delicate interplay. The gallium nitride acts as a host for oxygen, which not only aids the reaction but also enables a self-healing mechanism for the catalyst, ensuring prolonged performance. Such dual functionality exemplifies the breakthroughs made in catalysis, as the team demonstrated that their system can run continuously for over a hundred hours without loss of efficiency, a significant improvement over competing technologies.
With a remarkable conversion efficiency of 61% of the generated electrons contributing to ethylene production, the Michigan artificial photosynthesis system positions itself as a game-changer. To put this in perspective, existing alternatives, such as a combined silver and copper catalyst, achieved a lower efficiency and durability, operating only for a few hours in less favorable conditions. The robust performance of the University of Michigan’s device lies in its unique design and the synergistic relationships between its components, allowing it to sustain output while continually improving.
Looking ahead, the research team’s aspirations extend beyond producing just ethylene. They envision the potential to create longer-chain hydrocarbons, including fuels like propanol, which could redefine energy transport and storage capabilities. By pushing the boundaries of what is possible in carbon-the transformation process, they aim to provide viable alternatives for sustainable transportation in future practices.
The development of this artificial photosynthesis system represents more than just a scientific achievement; it embodies a fundamental shift in how we conceptualize CO2—not merely as waste, but as a resource for building a sustainable future. As research in this area continues to advance, the potential to harness and transform carbon emissions into valuable products could play a crucial role in combatting climate change. The ongoing work at the University of Michigan stands as a beacon of hope, illustrated by its impressive initial outcomes and ambitious future goals. As industries seek ways to achieve sustainability, innovations like this will undoubtedly play a pivotal role in shaping our energy landscape.

Leave a Reply