Helices are ubiquitous in the realm of biochemistry, serving as fundamental structures within various biomolecules, particularly proteins. Their helical formations are not merely structural curiosities but are pivotal in influencing how proteins perform their complex functions within living organisms. The twist and turn of a helix are dictated by its constituent amino acids, which are the building blocks of peptides and proteins.
Peptides, which are shorter sequences derived from proteins, exhibit helical properties due to the repetitive arrangement of amino acids. This specific configuration is crucial, as it creates a distinct three-dimensional shape that can affect how these peptides interact both with each other and with other biological molecules. The configuration is further complicated by chirality—a property that refers to the asymmetry of these structures, influencing their interactions and biological activities. An understanding of how these chiral characteristics contribute to the overall structure of peptides can provide insight into the molecular mechanisms that underpin their functionality.
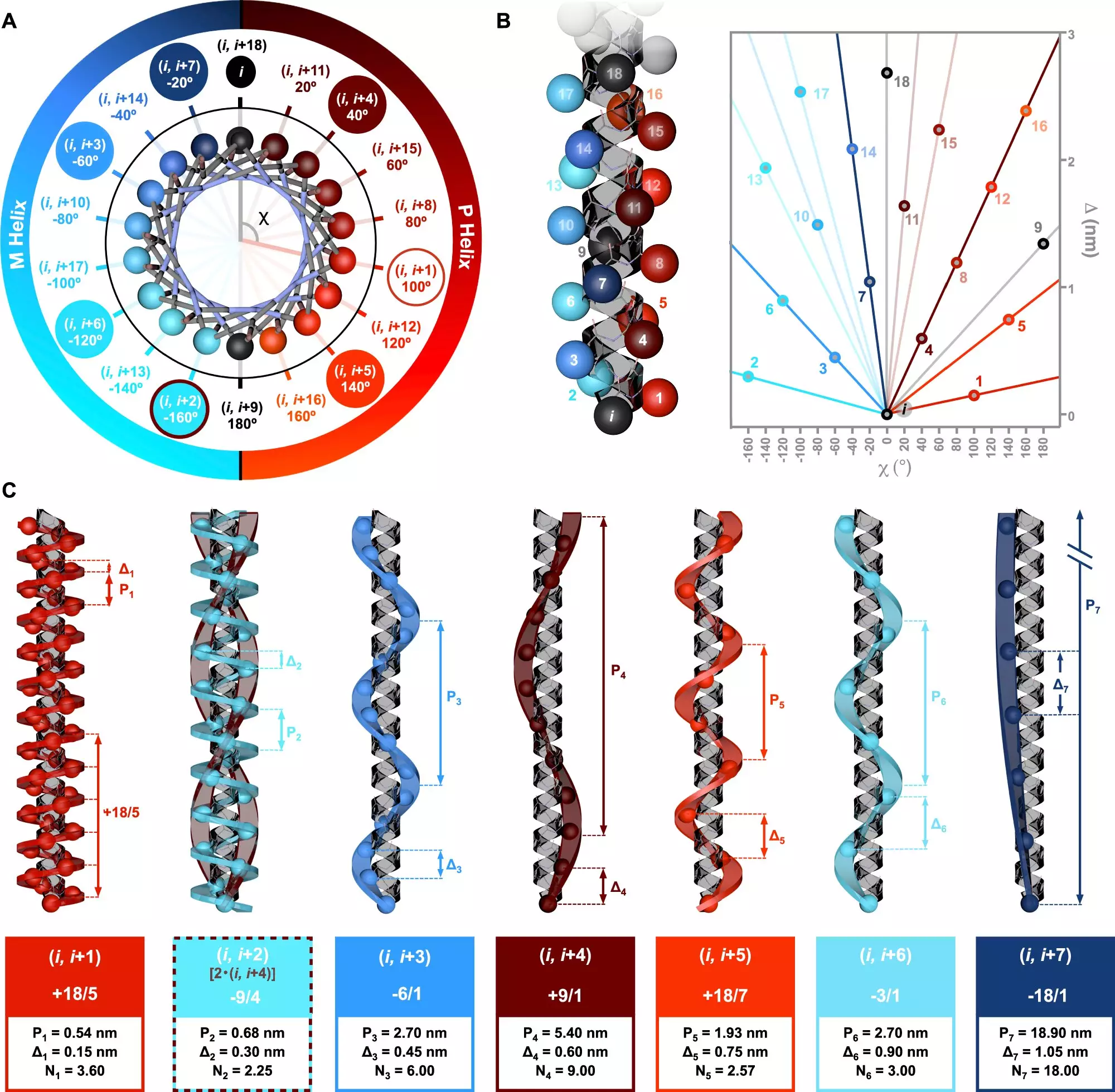
A significant contribution to this area is a study published in Nature Communications, led by Dr. Julián Bergueiro from the Center for Research in Biological Chemistry and Molecular Materials (CiQUS). This research unveils a sophisticated model of symmetry that elucidates how various amino acid sequences can lead to the formation of helical structures, paving the way for a deeper understanding of peptide chemistry. By exploring how distinct sequences yield diverse types of helices, this study underscores the complexity and variability inherent in molecular biology.
The research employed state-of-the-art computational techniques alongside circularly polarized light spectroscopy. This innovative combination allows for a nuanced analysis of how molecular structures respond to chiral light, thus revealing insights into their helical configurations. The experiments conducted provide empirical data that aligns well with theoretical models, demonstrating the potential of this approach in characterizing the diverse topologies that helices can adopt.
The findings from this research hold considerable promise for the fields of biotechnology and medicine. By manipulating the sequence of amino acids, scientists can potentially design peptides with specific helical structures tailored for particular functional roles. This capability could lead to the creation of novel therapies, biocompatible materials, and biomolecular tools that possess enhanced efficacy and specificity. As such, the study not only marks a significant advancement in peptide chemistry but also opens up new avenues for future research and application.
The ability to control helical structures at the molecular level represents a paradigm shift in macromolecular engineering. It highlights the intricate relationship between molecular structure and function, emphasizing how subtle changes in arrangement can lead to significant variations in biological activity. As we continue to delve into the complexities of helical formations, we edge closer to mastering the art of biomolecular design, harnessing nature’s own strategies for innovative applications.

Leave a Reply